The usefulness of inner ear magnetic resonance imaging in patient with Ménière’s disease: A narrative review
Article information
Abstract
Ménière’s disease (MD) is a multifactorial disorder with typical symptoms of recurrent vertigo, tinnitus, fluctuating hearing loss, and sensations of ear fullness. This disease greatly reduces the quality of life for patients. Unfortunately, it is difficult to diagnose and predictthe prognosis using only diagnostic methods, including audiometry. Therefore, since the mid-2000s, various efforts have been made to directly identify endolymphatic hydrops (EH), a histologic hallmark of MD, through magnetic resonance imaging (MRI) of the inner ear. Various studies have revealed significant correlation among degree of EH on inner ear MRI, patient symptoms, and test results. Although there are some limitations, inner ear MRI is expected to be widely used for differential diagnosis of MD, recurrent low-frequency hearing loss, non-specific vertigo, and vestibular migraine. In addition, as an automated analysis system of EH using the convolutional neural network algorithm has been developed,the usefulness of inner ear MRI is increasing. This algorithm can generate results that are highly consistent with those generated by manual calculation and can do so more quickly. Although there are some limitations to be overcome, inner ear MRI is expected to be widely used for differential diagnosis of various EH-related diseases in the not-too-distantfuture.
INTRODUCTION
Ménière’s disease (MD) was named afterthe French physician Prosper Ménière (1799 to 1862) at the end ofthe 19th century. MDis characterized by (1) sudden vertigo attack,(2) fluctuating sensorineural hearing loss, (3) tinnitus, and (4) aural pressure or fullness. The name MD is believed to have been derived from Adam Politzer’s use of the name ‘Symptom der Meniereschen Krankeitform’ in 1867 [1].
Although MD was first discovered by Prosper Ménière, he did not provide accurate pathological findings [2]. In 1938,the Hallpike et al. [3] and Yamakawa [4] group almost simultaneously published pathophysiological evidence of MD. They described endolymphatic hydrops (EH) in temporal bones of patients with symptoms of MD. Their findings were confirmed by several different histopathological observations, and EH is almost universally accepted as the most important pathological manifestation of MD(Fig. 1)[3,4].

Endolymphatic hydrops (EH), a histologic marker of Meniere's disease. EH increases the endolymphatic space, which can affect the inner and cause symptoms. Progression of MD is associated with progression of EH.
The globally accepted diagnostic criteria have changed several times. In 1972, ‘A hearing balance subcommittee under the American Academy of Otolaryngology’ set definitions and diagnostic criteria, and after that, the ‘American Association of Otolaryngology-Head and Neck Surgery (AAO-HNS)’ changed the criteria in 1985 and 1995 [5]. The most widely used diagnostic criteria today, created by a committee of the Bárány Society in 2015, have removed the “certain” and “possible” MDs (Table1)[6].
Results from epidemiological studies of the prevalence of MD vary by race or region, with reports ranging from 17 to 513 cases per 100,000 people [7]. The age of onset varies from 20 to 60 years of age, but the highest prevalence is seen in the 40s [8]. Such diversity in prevalence is due to racial and genetic differences, but at the same time, due to the lack of objective and definitive diagnostic methods [9]. Currently, patients’ symptoms are very important for the diagnosis of MD, and the only objective test other than that is pure tone audiometry (PTA).
The most objective method for diagnosing MD is to identify EH as a histological hall marker in the cochlear or vestibule, but it has been impossible to identify it for a long time directly. However, epochal changes began to occur with the development of magnetic resonance imaging (MRI) and post-image processing protocols for MRI in the mid-2000s [9].
At present, many groups have reported the study results for EH visualized by intratympanic (IT) or intravenous (IV) injection of a contrast media in MRI. Accordingly, MD-related research, which has been somewhat stagnant for the past several decades, is entering a new phase. Therefore, we summarize the usefulness of inner ear MRI in the diagnosis and treatment of MD.
THE HISTORY OF INNER EAR MRI FOR MÉNIÈRE’S DISEASE
Since the mid-2000s, various attempts have been made to identify EH in humans with the development of imaging technology using MRI. In 2004, Duan et al. [10] first successfully identified EH in the inner ear organ using 4.7 T MRI in live guinea pigs. The Nakashima group at Nagoya University in Japan confirmed EH after IT and IV injections of contrast media to MD patients using 3T MRI [11]. Since then, efforts to confirm EH using MRI have increased worldwide. In particular, IV-gadolinium (Gd)-enhanced inner ear MRI has several advantages over IT methods [12,13]. The IV contrast enhancement method is less invasive and much more efficient than the IT method (because it requires less time after contrast injection (4 hours vs. 24 hours) In addition, the IT method requires injection of both ears in order to evaluate both sides simultaneously, but the IV method can evaluate both sides simultaneously with a single IV injection [14]. Several studies have reported an association between the severity of EH on MRI and symptoms in patients with MD, and IV-Gd inner ear MRI is useful in diagnosing MD by showing a correlation between hearing-vestibular outcome and hydrocephalus. Several studies have reported an association between the severity of EH on MRI and symptoms in patients with MD. These results prove that IV-Gd inner ear MRI is useful for MD diagnosis by showing a good correlation with the previously used audio-vestibular tests [15]. Recently, more detailed and extensive studies are underway beyond simply comparing the EH seen in MD-MRI with various existing results. In particular, studies are underway to accurately determine the degree of EH by dividing the cochlea and vestibule into compartments or implementing a 3D model[15,16].
CURRENT DIAGNOSIS AND DILEMMA OF MÉNIÈRE’S DISEASE
The diagnostic criteria for MD proposed by AAO-HNS have been widely used and recently were revised in 2015 (Table 1). However, the changed diagnostic criteria are entirely dependent on subjective symptoms, except for PTA, and other vestibular diseases must still be excluded.
In addition to PTA, electrocochleography (EcoG), video head impulse test (vHIT), vestibular evoked myogenic potential (VEMP) test, and the caloric test did not belong to the diagnostic criteria, they have been widely used as additional tests for diagnosis. Among them, EcoG has been the most widely used as an adjunct to MD diagnosis for over 30 years [4]. However, since EcoG cannot directly visualize the endolymph space and EH, the limitations are clear, and the diagnostic value of this test is still controversial in severalreports and literature [17]. Several studies have found no significant correlation between EH levels and the interaural difference ratio in cervical VEMP [18,19]. However, some studies have shown that MD progression and VEMP are related based on the decreased amplitude of cervical VEMP in the lesion side of the MD compared to the contralateral ear[20].
Canal paralysis is frequently observed on calorie tests in patients with MD. However, even with canal paresis, vHIT often shows normal findings. It was hypothesized that this was not because the function of the lateral canal was actually decreased, but because the convection was not occurred well due to the expansion of the endolymphatic space inside the lateral semicircular canal [21]. However, recent studies using human temporal bone specimens and inner ear MRI suggest that this is due to invasion of the saccule or utricle into the canal rather than the expansion of the endolymphatic space within the canal [22]. Likewise, evaluation of the endolymphatic space through MRI supplements several limitations of existing tests and is still being developed.
USEFULNESS AND FUTURE OF MRI IN DIAGNOSIS OF MÉNIÈRE’S DISEASE
With the development of technology, MRI has higher resolution, high intuition and convenience, and involves no radiation exposure. Therefore, the use of MRI is expected to increase further in medical diagnosis and follow-up observation. In particular, inner ear MRI, which can directly confirm EH, is getting attention as an innovative test method for MD, which lacks an objective diagnostic method. Clinically, several grading systems have been introduced for reading and diagnosis,the most widely used of which was published in 2009 by Nakashima et al.[23](Table 2).
IV contrast-enhanced inner ear MRI has been widely recognized for its convenience and accuracy worldwide, and its scope of application is being extended beyond MD to the entire category of “Hydrophic Ear Disease” [24]. As summarized in Table 3 [13,25-44], many researchers have demonstrated that inner ear MRI shows a significant correlation with conventional tests, and the endolymphatic space seen on MRI is consistent with histopathological findings of temporal bone specimens of MD patients [22]. However, there are some limitations to measurement of EH using inner ear MRI. The first is that the image analysis process takes excessive time and effort and requires expertise. Second, since the resolution of MRI is not very high in 3T, errors can occur. Therefore,our group developed a deep learning algorithm using a convolutional neural network (CNN) for the analysis of EH (Fig. 2). Using this algorithm, quantitative analysis of EH in inner ear MRI proceeds quickly and conveniently, and the accuracy is very similar to that of an experienced radiologist or otolaryngologist. Specifically, compared with that measured by experienced physicians, the average interclass correlation coefficient (ICC) for all cases was 0.953; the average ICC of the vestibules was 0.968, and that of cochleae was 0.914. The time required for the fully automated system to accurately analyze the EH ratio in one patient’s one patient’s MR image stack stack was approximately 3.5 seconds [45]. Although further research is needed, it is likely that an algorithm using inner ear MRI and CNN will play an important role in the diagnosis of MD in the near future.
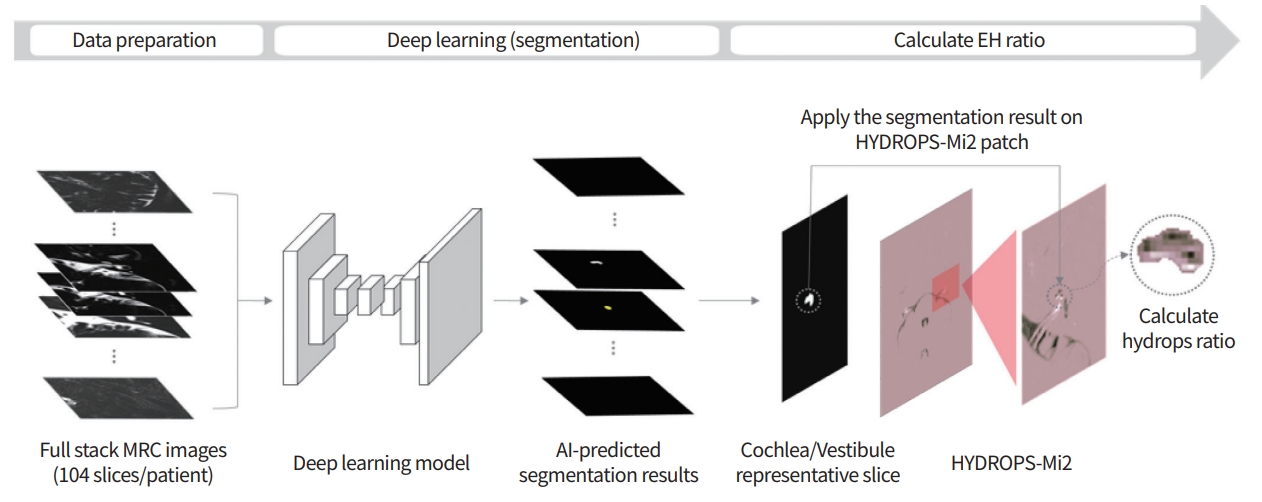
Process for calculating the endolymphatic hydrops ratio using a convolutional neural network. EH, endolymphatic hydrops; MRC, magnetic resonance cisternography; AI, artificial intelligence; HYDROPS-Mi2, HYDROPS (HYbriD of Reversed image Of Positive endolymph signal and native image of positive perilymph Signal) image Multiplied with heavily T2-weighted MR cisternography.
CONCLUSION
MD is one of the most difficult diseases to treat because it is difficult to diagnose, and the prognosis is not easy to predict. However, with recent development of MR equipment and the efforts of various researchers, an inner ear MRI protocol has been developed to directly view EH, simplifying clinical access. Although additional efforts such as research using deep learning are needed, the importance of inner ear MRI is expected to increase in various diseases including MD.
Notes
No potential conflict of interest relevant to this article was reported.
AUTHOR CONTRIBUTIONS
Conception or design: YSC, WHC.
Acquisition, analysis, or interpretation of data: YSC, BS.
Drafting the work or revising: YSC, WHC.
Final approval of the manuscript: BHC, WHC.
Acknowledgements
This research was supported by the Bio & Medical Technology Development Program of the National Research Foundation of South Korea (NRF), funded by the Ministry of Science and ICT (NRF-2020R1F1A1073904).



