 |
 |
- Search
| Precis Future Med > Volume 6(2); 2022 > Article |
|
Abstract
The Bonghan system (BHS) was discovered in the 1950s by Dr. Bong Han Kim in North Korea. His first report, published in 1962, revealed the identity of ‘acupuncture meridian’ as a vascular system. Kim published five reports, containing completely new facts on BHS: its distribution throughout the entire body, even in blood and lymphatic vessels and self-renovating function via a new cell cycle, demonstrating its fundamental nature in life. In about 1966, Kim’s research abruptly ceased but in about 2000, it was revived by Dr. Kwang-Sup Soh in South Korea, who later gave it another name, primo vascular system (PVS). Soh and other PVS scientists also uncovered new BHS/PVS facts: e.g., its roles in stem cell productions and in cancer metastasis. The review provides a glimpse of BHS/PVS science, which is so worthy of furthering. It includes: BHS and acupuncture meridian; BHS subtypes; sanal (산알)-cell cycle for cell-renovation and blood cell productions; sanals and stem cells; and cancer associated-PVS. The bases of BHS/PVS sciences are now laid out in front of us and it is up to us to combine our efforts together to further this important science. The review invites scientists in all fields to active debates to move forward and implement BHS/PVS sciences in healthcare.
Throughout the human history, many important scientific discoveries, including the famous Galileo’s revelation of ‘the Copernican system,’ have been intermingled with the politics, religions, particular personal or group’s interest, professional jealousy, etc. The Bonghan system (BHS)/primo vascular system (PVS) has experienced several rounds of this type of entanglements. A more detailed historical review on the BHS/PVS may be found in an article by Kang [1]. Briefly, Dr. Bong Han Kim graduated from the Medical School of the Kyung-Sung University (currently the School of Medicine, Seoul National University) in South Korea [2]. It is not very clear when and why Kim went to North Korea (North of the 38 parallel, then). After the Korean War was over in 1953, the Korean peninsula was permanently divided into two, by the Korean DeMilitarized Zone. Since then, no one was allowed to travel or communicate with the people between two Koreas. Considering that he was married and had children in South Korea, he might be one of many unfortunate people who had to remain in North Korea due to this permanent division.
After the end of the war, the leader of North Korea, Kim IlSung, declared ‘self-reliance (주체)’ in all aspects of national affairs, including medical practices. Even in Kim’s 1st report [2], the president of the Academy of Medical Science of North Korea emphasized the traditional Korean medical knowledge in the books of Dongeuibokam (동의보감, in Korean) by Joon Huh published in 1610 and Acupuncture and Moxibustion Experience (침구경험방, in Korean) by Im Huh in 1644.
It is also unclear why and when Kim started to work on the meridian system, as a medical doctor who had been trained for the western medical practice. During 1956 to 1961, Dr. Bong Han Kim, head of the chair of the physiology at the Pyongyang Medical College, was said to orally present his discovery of the acupuncture meridian (Kyungnak; 경락, in Korean) as a vascular system, for the first time in history, in a few North Korean scientific congresses. The content of his discovery was officially published in 1962, in a report titled ‘The substance of Kyungrak system,’ to the North Korean government [2]. For his accomplishment, he received a prestigious ‘The People Prize’ from the government, as well as the glorious remarks by Premier Il-Sung Kim. In the report, the very first optical image of the system [2] was presented. Kim’s study results clearly confirmed this newly found vascular system was different from the nervous, blood or lymphatic systems. In the Kim’s 2nd report, Kim’s photograph [3] was placed on its first page and the subunits of the system started to appear with Kim’s first name: ‘Bonghan corpuscle (BHC)’ for its node-like structure; ‘Bonghan duct (BHD),’ for the vessel(s) attached to BHC; and ‘Bonghan liquor (BH liquor),’ the liquid flowing inside the system. His official title was then the chief of ‘The Kyungrak Research Institute.’ The term ‘The Bonghan duct system’ representing the ‘Kyungrak system (i.e., acupuncture meridian)’ appeared in his 3rd report. Currently, in the scientific community dealing with this system, its short name ‘The Bonghan system (BHS)’ is commonly used, and to avoid confusion, this short term is used in the review, from here on.
After revealing the acupuncture meridian as a vascular system in his first report, four additional reports were published and they held massive and completely new scientific information. These reports were translated in several languages, printed in color on high quality papers, and sent out to other countries. Although many international scientists congratulated Kim for his accomplishments at that time, several scientists dismissed his discoveries, possibly because they could not repeat what Kim had done or simply did not want to give Kim the credit for discovering the very essence of the ‘acupuncture meridian.’
In around 1966, the North Korea experienced a power struggle between two top leaders, and Bong Han Kim and his institute experienced untimely demise as a collateral damage. The institute was shut down, BHS research in the North Korea was completely stopped, Kim disappeared, and all publications associated with BHS were confiscated and destroyed. However, because four of Kim’s reports had already sent out to other countries before the turmoil, these reports outside the North Korea survived. A copy of Kim’s first report in English [2] was located at the National Library of Australia; the Kim’s 2nd report in English [3], at the Duke University library in the United States; and the 3rd/4th reports in English [4], at the Berlin University library in Germany. More copies are expected to be found at various libraries in the world. The 5th report might not have an English version or sent out to other countries because it was published shortly before Kim’s disappearance.
Kim’s reports are called ‘reports,’ or ‘papers’ for lack of a better word, but each volume, except for his 5th one is, in fact, is a book with tens of sections, each of which contains the information sufficient to constitute a single research article. The titles of Kim’s five reports are:
Report 1: The great discovery in biology and medicine: substance of Kyungrak [2]; total 26 pages with 9 figures
Report 2: On the Kyungrak system [3]; total 41 pages (56 pages including pages with photos) with 38 figures and 6 tables
Report 3: Kyungrak system [4]; total 67 pages (approximately 100 pages including pages with photos), with 53 photos, 14 figures, and 10 tables
Report 4: Theory of sanal [4]; total 36 pages (approximately 60 pages including the pages with photos), with 34 photos, 5 figures, and 10 tables. Note that the reports 3 and 4 were published in a single volume.
Report 5: ‘혈구의 봉한산알-세포 환’ [5] (Bonghan sanal-cell cycle of blood cells, in English translation); total 8 pages, with 8 photos, 1 figure, and 4 tables; Unlike other reports being independent volumes, this report was published as one of the articles in a North Korean journal ‘Chosun Medicine.’
These reports might not be easily available. In 2010, during the 1st Symposium on the Primo Vascular System, ‘International Society on Primo Vascular System (ISPVS)’ was founded by Dr. Soh and the author, with selected international founding members. In around 2012, the website for the society (www.ispvs.org) was developed. In about 2013, the English versions of Kim’s first four reports were placed in the website to be available for general public, with a kind permission of Drs. Byung-Cheon Lee and Hoongi Kim, who had located them and provided the society with their copies in pdf format. It should be noted that ISPVS was able to do so because these volumes were not copyright protected. In around 2016 the responsibility of maintaining the site was transferred from the author to Dr. Soh. Since Dr. Soh’s passing, the site appeared to be ill-managed. Nevertheless, it still includes Kim’s reports, under the menus of ‘PUBLICATION/ARCHIVES’ → ‘archives BH Kim’s work’ → ‘Bong Han Kim’s publications.’ English version of Kim’s 5th report was not located or may not exist. It was, however, translated in English and published in 2016 [6].
In around 1965, a Japanese medical scientist Dr. Fujiwara in Osaka, Japan, pursued the BHS research and successfully identified the system [7]. Unfortunately, the Tokyo University medical scientists would not accept his findings and concluded that what he found were blood clots. No further research appeared to have been reported in Japan since then.
In about 2000, Dr. Kwang-Sup Soh, a theoretical physicist and professor of the Seoul National University, successfully identified BHS in animal models. He was, however, having difficulties in identifying the system with a good repeatability and, thus asked Dr. Fujiwara for help. With Fujiwara’s help, the Soh team was able to identify BHS more consistently [8]. According to personal communication with Dr. Soh, he soon realized that the research funding agency in South Korea appeared to feel uncomfortable for funding the research initiated in North Korea. In 2010, shortly before the first International Symposium on the Primo Vascular System [9], Soh renamed the BHS as PVS. He explained that the term ‘primo’ was adopted to signify the early formation of the system during the developmental stage, even before the blood vessels or the nerve; and also the Bong Han Kim’s belief of the system being the most essential system for the regeneration of organs and general health [10]. Now, there are three different names on a single system and, for readers’ convenience, Table 1 lists the terms of the acupuncture meridian traditionally used in Korea, those of BHS and PVS. Soh’s most important contributions, out of many, to the BHS/PVS science are: reviving the BHS research and verifying some of the Kim’s findings, including identifying the BHS subsystems; educating/training scientists both in and out of Korea, by sending his team to whomever asked for help, sometimes, with his own research funds; and discovering unique behaviors of cancer associated PVS (cancer-PVS). It is the author’s greatest regret to inform the readers that Dr. Kwang-Sup Soh passed away on November 10, 2021, while the author was preparing this manuscript. Journal of Acupuncture and Meridian Studies, of which Dr. Soh had been the editor-in-chief until 2018, placed an obituary in its 2022 issue (https://www.journal-jams.org/journal/view.html?doi=10.51507/j.jams.2022.15.1.1).
Until around 2010, the BHS research outside Korea was rare, perhaps with an exception of Fujiwara’s work. The author first met Dr. Kwang-Sub Soh in 2007 and learned about the system. Soon, two of her colleagues, Drs. Samuel Achilefu (then, Washington University; currently, the University of Texas Southwestern Medical Center) and Donald Miller (then, Director of the Brown Cancer Center, University of Louisville) became to learn about the system, particularly the cancer-PVS. In 2010, these two scientists and the author participated in the first International Symposium on Primo Vascular System [9]. All three scientists soon started the PVS research with help of Dr. Soh. Slightly different from the other United States BHS/ PVS scientists listed above, Dr. Vitaly Vodyanoy at the Auburn University in Auburn, AL, had been aware of the system from Kim’s reports sent to Russia (translated in Russian), and he started BHS research earlier than other United States scientists, also with Dr. Soh’s help. Vodyanoy’s major contributions to the BHS/PVS science are: ultra-high resolution optical imaging [11] and characterization of the BHS/PVS and its subunits; ‘sanal’ identification, purification, imaging; real-time imaging of the sanal’s unique movement in the liquid media and their merging (fusion) phenomena; and characterizing PVS microstructure and cells expressing various biomarkers, including stem cell biomarkers, inside primo nodes (PNs) of the bone marrow.
A large portion of BHS/PVS studies reported since 2000 was performed by the Soh team, and many of them are for identifying the system in animal models and thus confirming some of the Bonghan Kim’s findings. The new important findings by PVS scientists are: the extension of Kim’s ‘sanal theory’ to the stem cell science; and the potential relationship among the cancer-PVS, cancer stem cell, and metastasis. Although the PVS scientists have validated parts of Kim’s findings, due to the sheer volume of Kim’s work, most of them are still waiting to be verified.
This review is organized by the subject of topics covered by the Kim team and PVS scientists. Due to the large volume of Kim’s findings and study results by PVS scientists, which might be more than 200 articles by now, the review contains only a limited amount of information and explanations. Therefore, it is strongly recommended for the readers to visit the Kim’s reports and PVS articles. In the review, the term ‘BHS’ is used for the information published before 2010; and ‘PVS,’ after 2010, or sometimes both, to minimize the confusion caused by multiple names for a single system. Please note that figures copied from Kim’s reports have a low resolution because they are approximately 60 years old; and some of the reports available to the author were not in their best conditions.
Speculating from the first section of Kim’s first report [2], Kim appeared to have started his ‘acupuncture meridian’ research with characterizing electrical signals generated or responded to the stimuli at the Kyunghyul (경혈; acupoint) and Kyungmaik (경맥; meridian). He reported electric resistance, electric potential, and the waveforms of the signals at various acupoints and compared them at neighboring locations without acupoints. When an acupoint was stimulated by needles, chemicals, or heat, the signal generated at the acupoint transmitted to the next one along the same meridian, but when the meridian was severed the signal did not transmit. The signal from the system was shown to be unique and different from those from the nervous system. Kim then uncovered, for the first time in history, that the ‘acupucture meridian’ was no longer mystical entity but a tangible ‘vascular system.’
Kim’s 2nd report [3] introduced names for this newly recognized vascular system. The acupoint became the ‘Bonghan corpuscle (BHC),’ the meridian, ‘Bonghand duct (BHD),’ and the fluid inside the BHD, the ‘Bonghan (BH) liquor.’ Kim also realized that the system was present not only in the skin and also inside the body, including inside the blood and lymphatic vessels. He named the one in the skin the ‘superficial system’ and the one inside the body, the ‘profound system,’ stating that the commonly known the acupuncture meridian (the superficial system) was only a part of the entire vascular system. Although the report was now more focused on characterizing the new vascular system using Western biomedical tools, it still included analyses of the signals transmitted through the acupuncture meridian. Acupuncture needles placed in the superficial BHCs were said to show unique movements. The Kim team then harvested rabbit skin tissues containing superficial BHCs, kept them in moist, and placed electrodes on the BHCs for their bioelectric responses. When the low frequency, direct current was applied to the skin at various temperatures, the electric potential changed at the BHC with unique waveforms at various periods, while the signals were not sensed on the location without BHCs. For animals, the signal intensity gradually decreased after the animal died. The signals were also measured, with the changes in the duration of the electrical stimuli application or with chemical stimuli (e.g., pilocarpine, acetylcholine). They concluded that BHC’s were excitable tissue by external stimuli, lasting tens of minutes, and the signal was transmitted to the BHCs connected by the relevant BHD at a speed much slower than the one through the nerve. When the colon was applied with cold or hot saline the signal at the BHC of the lateral side of crus changed, but there was no change when the stimulus was applied to the small intestine.
In their 3rd report [4], although not extensively, the Kim team still reported bioelectrical characteristics of intra-vascular BHDs of profound BHS, while for their previous BHC studies, they had used mainly superficial BHCs. The BHD also showed electrical activities, while the signal changed very slowly. They observed the reactions of the heart, intestine, contraction of skeletal muscle, spinal reflux time, peripheral nerve, motor nerve, when the BHD was stimulated or severed. The functions of organs and tissues were found to be significantly affected by stimulation or severance of BHDs.
In Kim’s 2nd report [3], the team started to report on tracing the meridian using radio-isotope tracer P32 (K2HP32O4 or Na2HP32O4) by injecting it into the selected acupoints (superficial BHCs) of the inner side of abdominal wall or on the femur of the animal model. After a predetermined time following the injection, the tracer dosimetry at BHCs along the relevant BHD (i.e., acupoints along a particular meridian line) or outside the BHD was measured. Fig. 1A and C, show the dosimetry in number, at the BHC’s along the relevant BHD and the locations outside of it, in the abdomen and femur of the animal model, respectively; and also when the relevant BHD’s were severed in the (Fig. 1B) abdomen and (Fig. 1D) femur, respectively. The green circles indicate the injection BHCs and the red lines are where the BHDs were severed. As can be seen from the dosimetry number, the tracer went through all BHCs along the relevant superficial BHS but not outside of it, implying that BH liquor flew only through the relevant BHD. When the relevant BHD was severed (Fig. 1B and D) the numbers at the BHCs immediately above or below the cut line are very small, implying that, the BH liquor did not go through the severed BHD. This result also confirms the superficial BHS is the acupuncture meridian, and BHCs of the superficial BHS are acupoints.
Fig. 2A and B are the actual radiograms imaged when the tracer was injected into a known acupoint (green circle) at a lower part of the abdomen and into a BHC in the femur of the animal model, respectively. These figures show that the points emitting the lights are the locations of acupoints along the relevant meridians in the abdomen and femur, respectively. Fig. 2C shows a radiogram image of the abdominal skin of a rabbit. After the tracer was injected at the BHC in the femur, the abdominal skin was harvested and then attached on a film. Both the relevant BHD (meridian line) and the BHCs (acupoints) along the BHD are clearly seen, re-confirming that BH liquor flows through only the relevant meridian (BHD) and its acupoints (BHCs), and thus superficial BHS is, in fact, the acupuncture meridian.
For the BH liquor transport paths in the profound BHS, Kim’s team injected the tracer into the extravascular (one of the profound BHS subtypes, to be explained later) BHC and measured the level of the tracer in the BHD and surrounding tissues, with and without cutting the BHD. The BH liquor was found to flow mainly via the BHD. The team also studied the tracer level in the BH liquor and in the artery when the tracer was injected into the BHD inside the blood vessel, into the arterial or venous blood. The tracer inside the BHD was retained in the BHD, but not the ones in the blood vessels.
In their 3rd report [4], Kim’s team studied how the BH liquor circulated within the BHS subtypes; and also to various organs and tissues, via the BHS inside the blood and lymphatic systems and they found that the intra- and extra-vascular of profound BHS subtypes were linked. The team concluded that the BHS was a multi-circulating system, in which the paths of the BH liquor were interlinked and integrated, while individual subtypes maintained a certain level of independence; and that the change in the BH liquor circulation could seriously influence the function of the body organs.
The research after 2,000 includes few studies associated with BHS bioelectrical properties, its responses to external stimuli, or tracing the BHD or BH liquor circulation paths, maybe because identifying the system in the animal model was the immediate priority, and because there have been few oriental medical scientists participating BHS/PVS studies.
As stated, Kim [2] officially announced the real identity (‘substance’ in Kim’s word) of the ‘acupuncture meridian’ as a vascular system in his first report. Kim’s first image of the system was, thus, the one in the skin and the basic units of the system were expressed in acupuncture terms, i.e., Kyungmaik, the tubular structure (Kim’s words) and the Kyunghyul, the node-like structure. Kim’s team clearly confirmed that the system was histologically and biologically different from nervous (e.g., no Schwann cells), blood, or lymphatic systems by thorough analyses on the system. Later, Ogay et al. [12] introduced a helpful diagram, comparing the BHD, blood, and lymphatic vessels.
Kim’s 2nd report [3] was published less than 2 years after the 1st one, but its content on the characterization of this new system was so extensive that one can easily imagine how much the North Korean government had been supporting Kim’s research. Considering the study was performed more than a half century ago and the Kim team was dealing with so small and optically translucent objects, the quality of the images and diagrams, as well as the data analyses is all outstanding.
The team quickly noticed that the BHS was also present inside the body. The ‘superficial BHS’ was named for the BHS in the skin and, thus acupoints and meridians were BHCs and BHDs of the superficial BHS. The BHS inside the body became the ‘profound BHS.’ The team also realized, first time in history, that the BHS was present even inside the blood and lymphatic vessels (i.e., vessels inside vessels). Inside a single BHD, there were a bundle of even smaller vessels, and they were named the ‘Bonghan ductules’ (BH ductules). Their diameter varied in the range of 10 to 50 μm. According to Kim, ductules sometimes expanded and their epithelial cells had long and narrow (rod) shaped nuclei, at a size range 12 to 20 μm. The intravascular BHDs were said to branch at their diverging points and enter into the brain or linked to the superficial BHC’s, implying the BHS subtypes were interlinked.
The team reported detailed properties of BHS, particularly for the superficial BHS, including its morphology, physiological biochemical and histological, and its electrical properties, as well as the cells and biochemicals inside the system. The unique properties of superficial BHC were its thick outer layer of smooth muscle; standing in the reticulum with a BHD only one side of the BHC; lustrous and transparent; soft and light yellow; oval shaped with its shorter and longer diameters, 0.5–1.0 and 1.0–3.0 mm, respectively; and surrounded by blood vessels.
The profound system was reported to be different from the superficial BHS in its morphology, and located inside and around the blood and lymphatic vessel, around the internal organ, and connected to the superficial BHC via BHDs. Profound BHCs were in a fusiform and both of its ends were connected to BHDs, unlike the superficial BHCs. It is soft and more transparent than the superficial BHC; and its shorter and longer diameters are in the range of 0.5–1.0 and 3.0-7.0 mm, respectively.
Kim’s 3rd report [4], published less than 2 years after the 2nd one, had more detailed and organized information on the system, particularly for the profound system. The term ‘Kyungrak system’ was slowly replaced with the ‘Bonghan duct system.’ The team finally confirmed that the system was distributed throughout the entire animal body; and classified it into subtypes by their locations; and characterized them thoroughly, one by one. The superficial BHS, already well characterized in the 2nd report, was explained even more in detail, and the intra-organic BHS was added as one of the profound BHS subtypes. The names of BHS subtypes slightly changed from the 2nd to the 3rd reports but the following six BHS subtypes appear to cover all subtypes that Kim mentioned.
(1) Superficial BHS: This subtype is in the skin and its distribution is the one frequently shown in the meridian map. The subtypes listed below are the profound BHS, i.e., inside the body, contrasted to the superficial BHS, which is in the skin.
(2) Internal BHS: It is present inside the blood or lymphatic system, floating. It is also called the ‘intravascular BHS.’ In the review, from here on, intravascular BHS is used to minimize the confusion caused by other similar terms.
(3) Intra-external BHS: This subtype exists freely on the surface of the organs, and thus often called ‘organsurface BHS,’ which is used in this review, from her on. Kim stated that, sometimes, several BHDs of this subtype were linked to a single BHC.
(4) External BHS: This subtype was said to run along but outside the blood or lymphatic vessels, or the nerve; or independent of vessels. The BHDs of this subtype connecting to the superficial BHC was said to be the superficial BHD.
(5) Neural BHS: It exists in the central or peripheral nervous system, also running through the spinal canal and branching out to the body through the space between two vertebrae.
(6) Intra-organic BHS: It is located inside the internal and other organs and its size was said to be much smaller than other subtypes (BHC size, 0.1 to 0.5 mm).
One more BHC subtype described in the 3rd report was the terminal BHC connected to the terminal BH ductule, both of which are supposed be inside an individual cell and thus even smaller than the intra-organic BHS. Kim’s concept of a closed loop of BH liquor circulation path was, thus: individual cells → superficial BHS → profound BHS → intra-organic BHS → terminal corpuscle → individual cells. In fact, Kim showed optical images inside cells when the terminal ductule was severed. He also reported that the tracer injected into the superficial BHC was seen in the nuclei of certain parts of ovary, and the tracer injected into the intravascular BHD was accumulated in the parenchymal cell nuclei of liver. Kim interpreted these results to be the existence of terminal BHS. This aspect of Kim’s findings has not been investigated by PVS scientists yet.
The 3rd report contains numerous optical and electron microscope images and schematic diagrams, as well as tables and figures, for characterizing individual subtypes. PVS scientists (mostly Soh’s team) have identified most BHS subtypes. Figs. 3-6 show Kim’s macroscopic schematic diagrams of BHS subtypes; and microscopic optical images PVS subtypes identified since 2000, for an overview.
Fig. 3 illustrates the superficial BHS/PVS. Fig. 3A and B are Kim’s schematic diagram of the superficial BHS, the first subtype discovered by Kim, in its standing (in Kim’s word) view and an actual optical image, respectively, with its structural details and surroundings, including the hair and tissues [3]. This subtype is known to be difficult to identify and only a few PVS scientists were able to identify it. Lim et al. [13] used hemacolor contrast agent to observe this subtype in the skin of the rat abdomen (Fig. 3C), and collocated PN’s with the known acupoints in the area. Fig. 4 shows the intravascular BHS/PVS. Fig. 4A is Kim’s schematic diagram of the intravascular BHS distributed in the body [4]; and Fig. 4B and C show PVS inside the blood [14] and lymphatic vessels (by Kang’s team, unpublished data), respectively. This subtype have been identified by many PVS scientists, although the ones inside the lymphatic vessels [15-20] are known to be easier to identify than the ones inside blood vessels [14,21,22] because lymphatic vessels and fluid are translucent, while blood contain large quantity of hemoglobin, a strong chromophore. Fig. 5 shows the organsurface BHS/PVS. This subtype is probably identified the most by PVS scientists [12,23-28], and therefore, it has been frequently harvested and used for further analyses. Fig. 5A and B are the schematic diagrams of this subtype distribution on the internal organ described by Kim [4] and the PVS identified on the intestine [12], respectively. Fig. 6 illustrates neural BHS/PVS. Fig. 6A is Kim’s schematic diagram of neural BHS distribution, on the brain tissue, the ones going through the spinal column, and also the ones branching out between two vertebrae. Identifying neural PVS [29-32] is known to be difficult. Fig. 6B is a PVS on the brain [32] and Fig. 6C, the one passing through the spinal canal [29]. Only a few intra-organic subsystems were reported by PVS scientists and one of them was on the surface of the endocardia [33].
Up to now, PVS have been identified at least in 17 organs in seven animal species, including the ones listed above and the ones in the fatty tissue [34], placenta [35], the umbilical cord [35], and chick embryo [36], although the system has yet to be mapped in the entire body. Up to now, the only PVS identified in the human body are the ones in the placenta and umbilical cord [35]. As the research gets advanced more PVS in the human body are expected to be identified.
As the BHS research progressed and its properties became better understood, the Kim team defined its subunits more clearly. Fig. 7 shows the images and schematic diagrams of BHS/PVS subunites, selected from both Kim’s BHS reports and PVS articles, to provide macro- and micro-scopic views [3,37,38]. Fig. 7A is a typical organsurface PVS harvested from a rat model, displaying a PN, and primo vessels (PVs) connected both sides of the PN [38]. Fig. 7B is a Kim’s schematic diagram of a BHD with a bundle of BH ductules inside [4]. The endothelial cells of BH ductule are known to have unusual shape and a phase contrast microscope image (in the circle) of the BHD side view (red dashed rectangle) clearly shows the rod-shaped nuclei of BH ductules [3]. The wall of a PV vessel is found to be porous, unlike blood or lymphatic vessels [39], and this property can beneficially be used for identifying the intra-lymphatic PVS [19,20]. Fig. 7C shows a microscopic view of an organsurface PN imaged by Vodyanoy, using his ultra-high resolution optical microscope [11,37]. Once the subPVs of a PV get into the connecting PN, they become convoluted to form small sinusoids (Kim’s words) at the ends (white stars in Fig. 7C). Fig. 7D is a horizontal view (in the direction of the white dashed arrow, in Fig. 7C) of the sinusoid in a green dashed circle in Fig. 7C [11,37]. It is filled with small, cell-like structures and also tiny, round bodies possessing black dots in the middle (pointed by green, diamond-head arrows), which appear be the ones Kim referred as Feulgen reaction positive granules, and later confirmed as DNA.
In Kim’s 3rd report, BHS subunits were analyzed in detail, both qualitatively and quantitatively, by figures, images, and schematic diagrams, and tables.
(1) For BHDs and BH ductules, they presented the general and individual subtype’s characteristics, including their endothelial cells, outer membrane, and inner content.
(2) For BHCs, they also described general and individual subtype’s unique properties, including their morphological, histological, chemical, biochemical, cellular characteristics inside and outside of them. An interesting property of BHCs of the intra-lymphatic and superficial BHS was that they had four different stages.
(3) For the BH liquor, the team quantitatively analyzed its compositions, including the elemental gas, chemical, and biochemical, nitrogen, sugar, lipid, free amino acids, free nucleotides, hormones, base and nucleotide compositions of DNA and RNA. Also unusually large amount of hyaluronic acid was noted in it. For example, the intravascular BHD kept 25 times more hyaluronic acid than that in the blood clots.
(4) Large amount of DNA existed in the BHS. The system, particularly BH liquor, contained various biochemical and cells associated with body’s immune system. Kwon et al. [40] reported the cells and biochemical inside PVS, although they called the system by another name, for an unknown reason.
(5) Another highly important discovery reported in the 3rd report is, a completely new, subcellular entity ‘Bonghan sanal’ (sanal, 산알; ‘live egg’ in Korean) for the first time in history, which became the sole topic of the Kim’s 4th report.
In terms of the evolution, Kim claimed that BHS existed in all animals, including the ones as a low level animal as hydra, and also in all plants, i.e., in Kim’s words, ‘in all multicellular organisms.’ His team, in fact, studied the existence of BHS in several animals, including domestic rabbits, dogs, Guinea pigs and frogs; and a plant sunflower, as well as making some observation in the human body, although they did not describe what observation they made in detail.
Kim’s team also reported the BHS formation during the embryonic development, by studying fertilized chicken eggs. At around 10 hours after the incubation started, pre-BHDs were formed; At 15 to 16 hours, pre-BHDs differentiated fast and formed the wall of pre-BHDs, while no other cells or organs, except for the germ layers, appeared. At around 20 hours, oval-shaped nuclei in the pre-BHD moved from the center to the walls of pre-BHDs, and endothelial cells were formed and then BH ductules. By 48 hours, BHD formation was completed, while blood vessel walls started to form at around 20 to 27 hours. Lee et al. [36] later reported the PVS formation in the chick embryo and approximately 24 hours of incubation, PVs at a diameter of 40 to 90 μm and oval shaped PNs at its diameter of 80 to 200 μm were formed.
Kim’s 4th report was dedicated to the ‘Bonghan sanal,’ and ‘theory of sanal for cell renovation.’ The BHS has not been widely accepted by scientists in general, when it was first discovered and now, 60 years later. The fact that the ‘acupuncture meridian,’ the core but mysterious entity in the oriental medical practice for thousands of years, was visibly identified as a vascular system was not easily accepted by the scientists neither in the East nor the West. Also, the fact this system was present not only in the skin as in the meridian map but also inside the body and distributed throughout the entire body made it even more difficult to convince scientists. Then, Kim’s even more surprising discovery of the ‘Bonghan sanal’ inside the BHS, and its role for ‘self-renovation’ (Kim’s words) did not help scientists believe its existence or importance. Even for some PVS scientists, until recently, Kim’s ‘sanal theory’ has not been taken seriously. However, a report, containing 10 tables presenting numbers with standard deviations, 34 photos, and five figures along with thorough explanations in 100 pages, is not too difficult for whoever chooses to read it to believe this science of Kim’s.
The report stated that ‘Bonghan sanals’ were present in the entire BHS but mostly inside the BHC. A sanal is a translucent, spherical entity, at a size of approximately 1.0 μm (approximately 1/10 of a regular cell size), and composed of ‘Bonghan sanalosome,’ ‘Bonghan sanaloplasm,’ and ‘Bonghan sanal membrane.’ Fig. 8 shows the electron microscope image of sanals Kim had reported in his fourth report (Fig. 8A) [4] and; an optical microscope image of sanals by the Vodyanoy team, after they were harvested from PNs, purified, and placed in culture medium (Fig. 8B) [37], following Kim’s protocol [4]. Although the sanals in Fig. 8A might have been slightly deformed during the electron microscopy preparation, they look similar to the ones in the optical image in Fig. 8B, in shape and size. At the center of each sanal there is a particle-like body, sanalosome, which is to be a chromosome according to Kim. The Vodyanoy team has confirmed it to be DNA [37], although whether it was a single chromosome or not has yet to be examined.
After investigating for several animals and also a plant, Kim concluded that all multi-cellular organisms produced new cells in two ways: (1) By mitosis, where a cell duplicates all of its contents, including its chromosomes, and splits into two to form two identical daughter cells. In this cell cycle, the DNA is disintegrated into chromosomes and released via fragmented nuclear membrane; and the chromosomes are duplicated inside the cell membrane. Therefore, Kim called this cell cycle the ‘intracelluar Bonghan sanal-cell cycle’; (2) By Kim’s new cell cycle: The DNA of a cell is disintegrated to form individual chromosomes, as the same way as in the first one, each chromosomes are encapsulated, and released from the fragmented nuclear membrane to become independent entities called ‘Bonghan sanals,’ The cell membrane then breaks out and sanals are released out of the cell. Kim named this process ‘sanalization.’ Sanals are then transported from the tissue to BHDs and then finally transported to BHCs via BHDs. In the BHC new cells are formed, and the author named this cell forming process ‘cellation.’ Kim called cycle of combined sanalization & cellation, the ‘extracellular Bonghan sanal-cell cycle,’ He claimed that chromosomes were, thus, sanalosomes, and the number of sanals produced from a single cell was the chromosome number of the species. After thorough investigations, Kim concluded that the transportation of sanals from the tissue (via BHDs) and cellation (in BHCs) happened only in the BHS, and therefore, sanals were rarely seen in the blood or lymphatic vessels. Fig. 9 describes the ‘extracellular Bonghan sanal-cell cycle,’ presented by Kim and the red line shows the sanalization and the blue, the cellation processes. In this review, from here on, a shorter term ‘sanalcell cycle’ is used for the Kim’s new cell cycle.
Kim noticed that isolated sanals in culture media having a unique movement, different from the Brownian motion, and he said that the movement was by the chromosome and it is sensitive to the media temperature. This incessant movement of sanals was also observed by the Vodyanoy team [37]. Kim’s team analyzed the composition of sanals, including mineral, amino acid, protein, nitrogen, sugar, cholesterol, hyaluronic acid, RNA, and particularly DNA. The amount of DNA in a single sanal was said to be equal to that of a chromosome and Kim’s calculation of the molecular weight of DNA in a single sanal was 1.8–3.0× 10-6. The base composition of DNA and nucleotide composition of RNA in the sanal were said to be the same as those of the cell, and BH liquor contained the nutrient for the sanal growth.
Kim’s report also teaches us how to produce sanals from a cell in vitro and Fig. 10A exhibits a series of sanalization process [4]. The nuclear membrane is loosened as sanals are formed (1, 2). These sanals move out of nucleus through the disintegrated nuclear membrane to cytoplasm (3, 4). The cell membrane is also breaking out and sanals are freed from the cell (5). Fig. 10A (A*) displays sanals escaping from the cell membrane (arrow) in a tissue sample [4]. Fig. 10B (unpublished data; permission obtained from the Vodyanoy team) is an optical image of white blood cells (in vitro) going through sanalization, after placing cells under stressed conditions by owering media temperature with limited nutrient. There are many tiny, round and translucent bodies in the media, appearing to be sanals.
The Kim’s report also teaches us how to isolate sanals from BH liquor, obtain a single sanal, and then cultivate it to grow into a cell (cellation) in vitro. The cellation process was said to be in two stages: (1) The stage of proliferation (Fig. 11A; 1–4): While a sanal is in a medium, moving in its unique manner, its sanalosome produces fine filaments, send them out of its membrane, and then produces daughter sanals. Both mother and daughter sanals produce more sanals but all are still bundled together by the filament, thus looking like grapes; (2) The stage of fusion: After the first stage, bundled sanals merge/fuse together and soon thin membrane-like structure is formed around the fused sanals, forming a nucleus-like structure. Then homogenous substances are formed around the structure, slowly constituting cytoplasm, and finally the cell membrane is formed (Fig. 11A; 5–9). Fig. 11B shows a series of sanal merging/fusing processes that the Vodyanoy team observed when purified sanals were placed together in media [37]. A certain number of sanals merged (1, 2) and form a dense structure (3) and a membrane-like boundary was formed around the structure (4). The experiment unfortunately stopped at this point.
A question on Kim’s cellation process is: how a single sanal with a single chromosome becomes a cell with full DNA sequence of the animal, although more studies are needed to fully understand the cellation process.
The Kim team also investigated the change in the biochemical content (nucleic acid, protein, amino acid) in sanals obtained from rabbit intravascular BHS, during the cellation process of 144 hours. At the end of the process, the amount of DNA increased 16 times; the RNA, nine times; and the protein nitrogen, 32 times. An interesting observation by the team was that the cellation progressed better with light application in the media.
After studying the sanalization and cellation processes of various animals and plants the Kim team concluded that all multi-cellular living-beings possessed sanals and their sanals were similar in shape and size. The team also performed continuous sanalization-cellation full sanal-cell cycles in vitro, for several different animals and a plant. Sanals harvested from the BHS of rabbit liver went through cellation process to produce liver cells; Sanals obtained via sanalization process of nerve cells from a rabbit brain formed axon cells after the cellation process; Sanals from chick embryo formed cells via the cellation process; Sanals from fertilized frog eggs were cultivated to produce cells; Sanals produced from leukocytes were cultivated to produce leukocytes; Sanalized sunflower root cells were successfully cultivated to yield cells; etc. The cellation process usually took 72 to 120 hours.
To observe the sanal-cell cycle in the normal and injured tissue, the Kim team used rabbit liver tissue. In the normal tissue, 2% to 4% of the cells were sanalized. If the animal were starved, sanal production increased. After the injury, the sanal-cell cycle became vigorous; and sanal production and cellation processes increased until new cell number reached to normal counts. Approximately 7 days after, new cells were formed and the cellation process in the rabbit liver was the same as those in the culture: Sanals first become nucleus-like structures at a size of 3 to 5 μm, with a minimal amount of cytoplasm, and then become cells. They found that 1% to 3% of normal liver cells were from sanal.
The team also traced the moving paths of sanals in the body by injecting radioisotope labelled sanals into the BHS or tissue of the internal organs and followed the tracer in the lung, liver, kidney, and ovary. Approximately 48 hours after the injection, matured cells with the tracer were found in all organs studied, but the ones injected into the blood were not grown. Tagged sanals injected to superficial BHC matured and went to internal organs, and then distributed into BHCs of various subtypes. The ones injected to BHD went to internal organs. The team also collected sanals from 79 acupoints to see if the sanals from these points produced the same cells. They found that the cells from the different acupoints produced the cells of different organs, relating to the acupuncture therapy utilizing a particular acupoint to treat diseases of a particular organ. They also reasoned this might be the reason their in vitro sanal culture was affected the light supply (i.e., superficial BHCs are exposed to the light more than profound BHCs?).
During the Kim’s time, the science or terms currently used in the regeneration science, such as stem cells, stem cell biomarkers, CRISPR, were not established, and Kim, thus, did not relate his sanal-cell cycle with these terms. Several PVS scientists have recently confirmed that sanals express stem cell biomarkers and cells inside PNs, appearing to be originated from sanals (in Kim’s sense, after cellation), express various stem cell biomarkers and behave like stem cells. Ogay and Soh [38] reported that sanals expressed stem cell biomarkers octamer-binding transcription factor 4 (OCT4) and NANOG Lee et al. [41] harvested small (3 to 5 μm) cells from intravascular PNs and found that these cells expressed stem cell biomarkers. These cells were, however, different from sanals that Kim or the Vodyanoy team described [4,37] in their size and characteristics, and they appeared to be the structure after the first stage of cellation, which Kim had reported as approximately 5 μm in size with little cytoplasm [4]. Lee et al. [41] properly differentiated these cells into neural cells in vitro, applied to the brain of the animal model with ischemic brain damages, and the brains were partially repaired. This team also studied whether their cells were the ‘very small embryonic-like stem cells (VSELs),’ which the Ratajczak team had discovered and been studied for a long time [42]. They concluded that their cells did not appear to be VSELs because of the slight differences in biomarker expressions and a few other properties.
Although more studies are needed to fully understand the sanal-cell cycle, if all multi-cellular organisms have stem cells then Kim’s claim of all multi-cell organisms having the BHS and the sanal-cell cycle appears to be reasonable.
Kims’s fifth report [5,6] was published on October 8, 1965, 6 months after the 4th one. The report deals with the production of blood cells, including red blood cells (RBCs), granulocytes, and lymphocytes via the sanal-cell cycle, both in vitro and in the BHS inside the regenerative organs, particularly those in the bone marrow and lymph nodes of rabbits.
The team produced sanals from nucleusless RBC (Kim’s term, NLRBC) and characterized their molecular and biomolecular compositions, including nucleic acid, nitrogen, hemoglobin, oxygen uptake rate, and fat, and also activities of cytochrome oxidase and catalase, during the cellation process. These sanals naturally had no sanalosome (i.e., no DNA), and were easily destroyed by the high and low temperature, and sensitive to the osmotic pressure. Approximately 96 hours after starting the culture, sanals were fully matured and became NLRBCs, without the stage of the nucleus formation, although RNA was still present in them. The maturation process of NLRBCs was rather difficult for the team to observe due to the presence of hemoglobin.
The team also observed the sanalization and cellation processes inside the BHS of their animal model. Sanalization of NLRBCs was observed in the sinusoids of superficial BHCs and the produced sanals were transported to intravascular BHCs via BHDs. Cellation of NLRBC was done mainly in the intra-blood vessel BHCs and the BHS in bone marrow.
Sanalization and cellation processes of granulocytes were reported to occur in the similar paths as NLRBC or other cell renovation process, in the intravascular and bone marrow BHCs. As the cytoplasm became matured; however, a small number of unclear, neutrophil granules appeared. The nucleus configuration was either not well defined or kidney shaped, and chromatin appeared hazy and dispersed. The cytoplasm and nucleus were then gradually formed to be typical, neutrophil granulocytes. Eosinophil granulocytes also underwent a similar maturation process.
For the lymphocyte, the sanalization was said to occur inside the BHCs of BHS and also in the lymphatic system; and the cellation happened inside the intra-lymphatic BHCs, lymph nodes, and in the external and organsurface BHCs.
In the blood cell producing organs, e.g., bone marrow, BHD sinusoids were together with blood vessels, forming network-like structures. Sanalization progressed inside the blood and lymphatic vessels and cellation occurred in the BHCs. The BHS structure inside the bone marrow was said to be unique in that the intra-blood vessel BHS protruded the blood vessel wall to access to the bone marrow structure, forming a beehive-like network. Some branched out to the inner surface of the bone. The BHDs coming out of the blood vessel formed a dense network and, inside the BH ductules, various stages of the cellation were being progressed.
The BHS in the lymph node were said to be similar to that in the bone marrow. They came into the node either via blood or lymphatic vessels, and formed beehive-like networks in the capsules, trabecula, lymphoid nodules, and medulla, which were independent of blood vessels or nerves. They also formed complex networks inside the lymph sinus, and were connected to the cell nuclei and immature nucleus-like structure.
To better understand the sanal-cell cycle of blood cells in vivo, the Kim team injected labelled RBC and lymphocytes into the ear vein of the animal model and traced the signal for 20 days. After 2 to 8 days, sanalization process of the labeled RBCs was observed in the sinusoids of superficial BHCs and both RBCs and RBC sanals were observed there. Inside the bone marrow, labeled RBC sanals were also seen. Inside the intravascular BHC, maturation process of the RBC, originated from labeled RBC sanals, was observed.
For the granulocyte study, a section of bone marrow was cultured and examined. In the bone marrow, various stages of the sanalization of the labeled granulocytes were observed and 10 to 12 labeled sanals were produced from a single labelled granulocyte. Also these sanals were matured to become white blood cells. Inside BHCs, maturation process of labeled granulocyte sanals were also seen.
For lymphocytes, sanalization process of labeled lymphocytes was seen in the white pulp of the spleen and also in some part of the red pulp. Labeled sanals became matured in the lymph nodes to become lymphocytes with dense, basophilic nuclei and with little cytoplasm. In the BH ductules inside the BHCs, labeled lymphocyte sanals were seen; and inside the net-like structure, fully matured lymphocytes were observed.
The Vodyanoy team has studied the PVS inside the bone marrow extensively [43,44], and many of their findings were similar to what Kim described on the BHS in the bone marrows. Fig. 12 shows two out of many sets of images that the team presented in their paper [43], Fig. 12A shows a PVS, being extracted from the bone marrow of a mouse model and Fig. 12B show images of sinusoids inside the PNs in the bone marrow, after reacted with antibodies against stem cell and other biomarkers, while the sixth (last) image, with no antibodies. The sinusoids are packed with many different sized, cell-like structures, expressing various biomarkers including stem cell biomarkers, and some of the rims of holes express the stem cell biomarkers of the cells, which used to be held there, implying sinusoids in the PNs are filled with stem cells. Hwang et al. [45] studied the cells extracted from PNs of the PVS from the vein, artery, and organ surface of mice. After they co-cultured the entire node components with OP9 cells (mouse bone marrow stromal cells derived) they found immature cells at a size approximately 5 μm with a high nucleus to cytoplasm ratio, which, again, appeared to be the structure at the first stage of the cellation process that Kim described.
When Kim’s team created anemic animal models, the overall appearance of the bone marrow looked damaged. Under this anemic condition, the size of the intra-blood vessel BHCs increased their size and number, the BHDs connected to them also expanded and the BHCs were filled with many RBC sanals. This phenomenon was also observed by PVS scientists. Lim et al. [27] and Shen et al. [28] showed the organsurface PVS of a rat model with anemic condition producing RBCs, with their size and number significantly increased. This implies that BHS/PVS subtypes communicate among themselves and when a part of body experiences an abnormal condition, PVS subtypes in other parts take parts in the role of the malfunctioning PVS, in an attempt to maintain body’s homeostasis, showing the remarkable plasticity of the BHS/PVS. At the end of his 5th report, Kim concluded that, as in other cell production, blood cell production was done both by the cell division and via the sanal-cell cycle, but most blood cell production was done via the sanal-cell cycle.
As listed above, Kim and his team had performed enormous amount of basic sciences on the BHS. What his team had achieved within less than 5 years would probably take tens of years for other scientists/teams just to repeat to verify. Considering the fundamental roles of BHS in the body, one might also speculate that malfunctioning BHS would cause serious illnesses. Kim probably had not had time to relate the BHS fundamentals to specific diseases associated with BHS malfunctions, during his relative short lifetime in this research, except for studying the non-marrow BHS producing RBCs during anemic condition.
As mentioned, one of the important accomplishments of the Soh team is investigating the behavior of PVS in cancerous environment. The team, for the first time, reported that PVS was unusually proliferated on the surface of xenografts of lung cancer (NCI-H460; Fig. 13A) [46] and ovarian cancer (SKOV-3; Fig. 13B) [47]. To better understand the role of the cancer-PVS in cancer cell transport, the Soh team used quantum dot (QD)-electroporated cancer cells for their xenograft studies (Fig. 13B). They reported that the QD emission level generated by the cancer cells moving through a PV from the primary to the secondary tumor (S2; solid arrow) was approximately twice of that via a lymphatic vessel to another secondary tumor (S1; dotted arrow). In addition, PNs of the cancer-PVS were filled with QD-light emitting cancer cells, indicating potential cancer-PNs producing/storing cancer cells, and cancer metastasis via the cancer-PV [47]. Other PVS teams in the United States also studied the cancer-PVS for various cancer xenografts and all observed the abnormally high density of PVS generated on the tumor. These xenografts are; cutaneous melanoma B16BL6 (Fig. 13C) [48]; mammary carcinoma 4T1 (not shown here) [49]; gastric cancer MKN12 (Fig. 13D) [50], where a translucent PV connecting the primary tumor (P) and secondary one (S); leukemia U937 (Fig. 13E) [51]; and breast cancer MDA-MB123 (Fig. 13F) [50]. When the Miller Group (Fig. 13E) [51] isolated the cancer-PVS on the leukemia xenograft to examine the origin of the cancer-PVS (i.e., either human cancer or animal model), their cancer-PVS was human-origin, not from the host. In addition, the cancer-PVS extensively overexpressed, stem cell specific transcription factors, particularly Krüppel-like factor 4 (KLF4), an upstream regulator of NANOG, implying the cancer-PVS might be the site of producing, storing, and transporting cancer stem cells.
Due to the lack of knowledge in the biomarkers specific only to the PVS, most cancer-PVS studies were done with PVS on the surface of the tumor (not inside the tumor), although Zhang and Oh [52] recently reported monoclonal antibodies developed for PVS, excluding blood or lymphatic vessels. In contrast to cancer-PVS studies, the Hendrix group [53] has been investigating unusual vascular structure called ‘tumor vasculogenic mimicry (TVM).’ inside the human melanoma for a long time. Since this group’s discovery of TVM in 1999, many other scientists have reported it being associated with metastasis of various malignant tumors and containing cells with stem cell properties. It may be worthwhile to investigate whether the cancer-PVS is related to the TVM or not. As biomolecules specific, only for the BHS/PVS became available, the study on the cancer-PVS is expected to more rapidly progress, as well as developing potential diagnostic and treatment tools for cancer and cancer metastasis.
The discovery of the Bonghan duct system 60 years ago and its thorough characterization by Dr. Bong Han Kim are undoubtedly one of the most important scientific achievements in history. Kim taught us that:
(1) The BHS is the tangible identity of the ‘acupuncture meridian,’ which had been the basis for the Oriental medicine and of which the practice had been mysteriously passed down from one generation to the next, for thousands of years;
(2) This system is distributed not only in the skin, as seen in the meridian map, but also inside the body, including the blood and lymphatic systems, indicating its fundamental nature for maintaining life;
(3) The system renovates cells via the ‘sanal-cell cycle.’
After 40 years of a dormant period of BHS research since Kim disappeared, fortunately, Dr. Kwang-Sup Soh revived the science, with a new name of PVS, and added new findings onto Kim’s. He also educated/trained scientists whoever wanted to learn about it. He opened a door to better understanding the relationship between the cancer-PVS and cancer metastasis.
New PVS scientists have been working on the relationship between Kim’s ‘sanal-cell cycle’ and the stem cell science, and potential role of the cancer-PVS in the cancer stem cell production and storage, and cancer metastasis.
As much as we learned about the BHS/PVS science from Kim, Soh, and other PVS scientists, there is still so much for us scientists to do, to fully understand the system, starting from verifying the enormous amount of what Kim had reported to furthering its science. However, the BHS/PVS history has shown us the struggles that Kim, Soh, and other PVS scientists have experienced and difficulties still remain for continuing its research: In terms of publications in reputable journals, most reviewers are unfamiliar with the system; some editors decided not to forward BHS/PVS manuscripts for the review or choose not to publish it even after reviewers accepted them. Many PVS articles, thus, tend to be published in a limited number of journals not well known to the scientists in general. Obtaining research funds has been difficult for the similar reason. Some program directors refused to fund a PVS project after the proposal had received a score within the funding level. Nevertheless, discontinuing the BHS/PVS research is not an option because this science is so important for improving human healthcare.
Because the field of BHS/PVS science is so huge that it is difficult for an individual to tackle all, especially when the research funds are scarce. A way for furthering this science may be forming a multi-disciplinary team/network with like-minded scientists, with individual groups taking particular topics of their specialties, and keeping tight communications among groups to avoid duplicating efforts. Because of the fundamental nature of the BHS/PVS in maintaining life, most of biomedical science disciplines may participate in this effort, including anatomy and physiology; stem cell sciences and regeneration; hematology and vascular sciences; oncology; biochemistry; genomics; proteomics; immunology; acupuncture and oriental medicine, molecular- and bio-imaging; biophysics, bioengineering; etc.
At the same time, applying already verified BHS/PVS knowledge for developing medical tools is important. An example may be developing techniques for producing sanals from one’s own cells and producing new cells, particularly stem cells, from these sanals, for treating diseases requiring new cells, e.g., blood and/or stem cell production from patient’s own cells, enabling personalized medicine. Another example may be developing tools for non-invasive detection of cancer-PVS for early detection, diagnosis, and treatment of cancer.
Most importantly, training junior scientists in basic tools for the BHS/PVS research is essential for keeping this science alive, considering the system is difficult to identify, due to its small size and optical translucency. Most PVS scientists have taken advantages of Dr. Soh’s generosity in training, after himself receiving Dr. Fujiwara’s help for achieving better science. An excellent way to train scientists may be opening BHS/PVS courses at the educational institute and Dr. Vodyanoy has been planning to have PVS courses at the Auburn University, AL, USA. Such an effort may also help general public be acquainted with this vascular system.
The author is proud to have the Korean heritage that produced Drs. Bong Han Kim and Kwang-Sup Soh. As a bioengineer and scientist, however, she wishes this review to have convinced the readers that the BHS/PVS science is bigger than an individual, a research group, or a country; and that the BHS/PVS research and its outcomes should be globally shared. It is also her wishes to open dialogues among the readers to share positive ways of furthering this important science.
Notes
AUTHOR CONTRIBUTIONS
Conception or design: KAK.
Acquisition, analysis, or interpretation of data: KAK.
Drafting the work or revising: KAK.
Final approval of the manuscript: KAK.
Fig. 1.
Dosimetry levels at various locations of the body of an animal model, after P32 was injected into an acupoint (Bonghan corpuscle [BHC]) in the abdomen or femur, and when the relevant meridian (Bonghan duct [BHD]) was retained or severed [3]. The dosimetry numbers at the acupoints (BHC) along the relevant meridian (BHD) or at the locations outside the relevant BHD in (A) the abdomen and (C) femur, respectively. The numbers at the BHCs along the relevant BHD are much greater than those at the locations outside the BHD. The numbers at the BHCs along the relevant BHD in (B) the abdomen and (D) femur, respectively when the relevant BHD was severed The numbers of tracer level at the acupoints immediately above or lower the severed location of the relevant BHD are much lower than those of the BHCs still connected to the BHD. Green circles denote the acupoints where the tracer was injected and the red lines are the location where the BHD was severed.
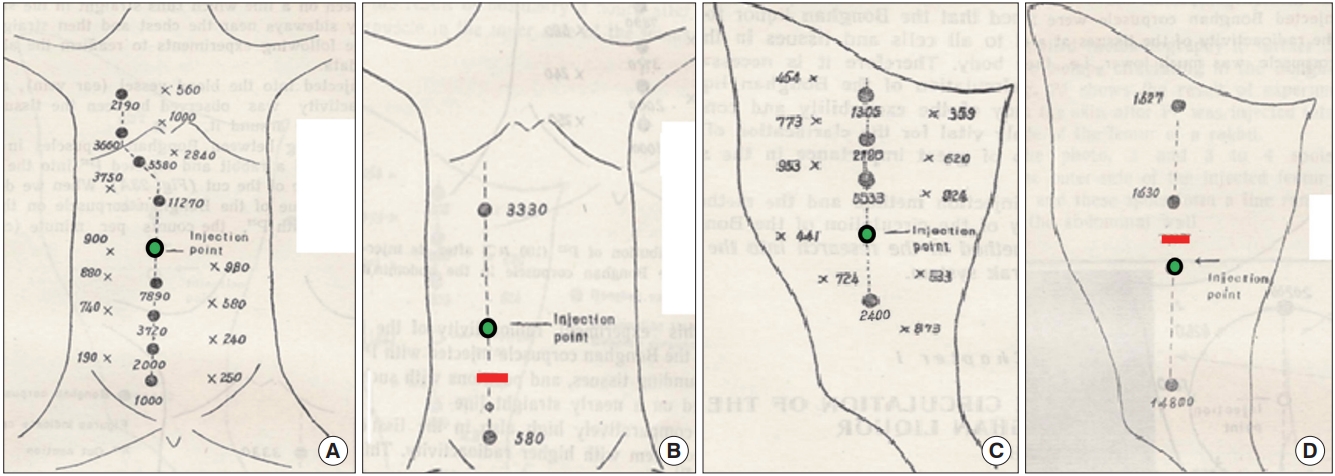
Fig. 2.
Radiograms taken from the abdomen of rabbit models after the tracer was injected into a Bonghan corpuscle (BHC; green circles) [3]. The acupoints (BHCs) along the relevant Bonghan ducts (BHDs) emit lights like shining dots, while no other location emits light, after the tracer was injected (A) into an acupoint of a lower part of the abdomen and (B) into an acupoint of the lateral side of the femur, respectively. (C) An image of the light emission from the skin harvested from the abdomen of a rabbit after the tracer was injected into an acupoint (BHC) in the femur. The harvested skin was attached to a film to obtain the image. Both BHCs and the relevant meridian (BHD) emit light, clearly revealing Bonghan liquor flowing through both the BHD and the relevant BHCs.

Fig. 3.
Superficial Bonghan system (BHS)/primo vascular system (PVS). (A) A schematic diagram of a superficial BHS with other anatomical structures around it, by the Kim team [3]. (B) An optical image of a superficial BHS, with detailed its subunits, by the Kim team [3]. (C) PVS in the subcutaneous tissue of the rat abdomen (hemacolor-stained), collocating primo nodes (PNs) with the acupoints along meridians (primo vessels [PVs]) [13]. Dotted, black line arrows point the acupoints (PNs) along the conception vessels (CVs).
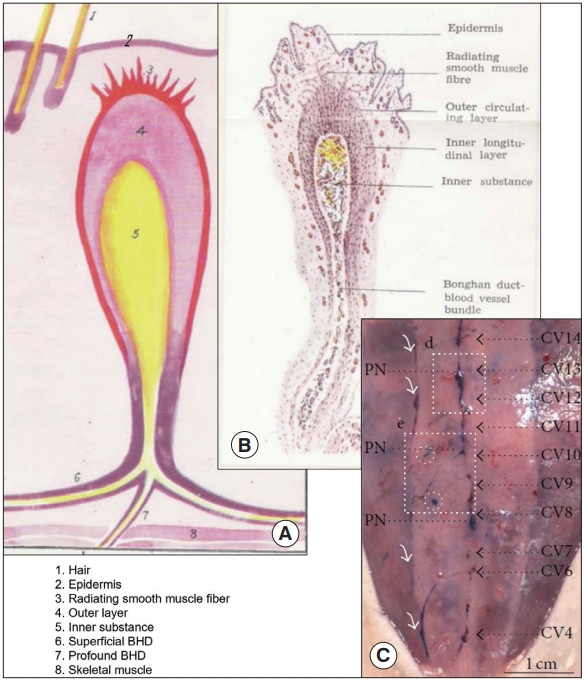
Fig. 4.
Intravascular Bonghan system (BHS)/primo vascular system (PVS). (A) A schematic diagram of the intravascular BHS distribution in the body [4]. (B) A PVS (white arrows) inside the inferior vena cava (black arrow) of a mouse, contrasted by Alcian blue, in vivo and in situ [14]. (C) A PVS inside a lymphatic vessel, contrasted by Alcian blue, imaged by the Kang team (unpublished data).
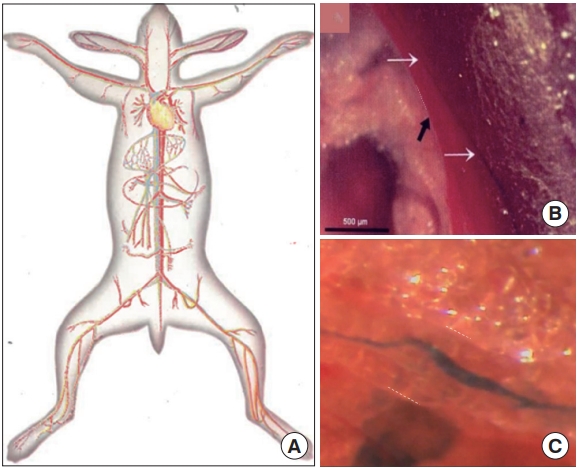
Fig. 5.
Organsurface Bonghan system (BHS)/primo vascular system (PVS). (A) A schematic diagram of the organsurface BHS distribution in the animal body, by the Kim team [4]. (B) A PVS on the small intestine of a rabbit, contrasted with methylene blue [12]. Thin lined arrows, primo vessels; and a thick arrowhead, a primo node.
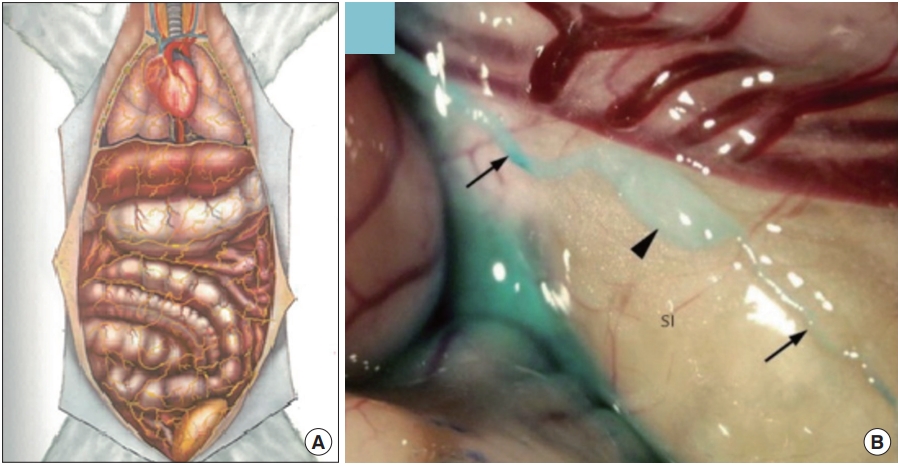
Fig. 6.
Neural Bonghan system (BHS)/primo vascular system (PVS). (A) A schematic diagram of the neural BHS distribution in the brain and spines [4]. (B) A PVS located in the brain (in the dotted blue circle), between left cerebrum and cerebellum. In the blue circle on the lower right corner is the enlarged image of the PVS marked by the dotted blue circle. Adapted from Dai et al. [30], with permission from Wolters Kluwer Medknow Publications. White arrows point primo vessels (PVs), and the blue arrow points a primo node. (C) A long PV, lifted by a thin needle, running from the brain passing through the spinal canal. The small black arrow on the upper right corner points a PVS on the brain, connected to this long PV [29]. SSS, superiaor sagittal sinus; TS, transverse sinus.

Fig. 7.
Subunits of Bonghan system (BHS)/primo vascular system (PVS) [37,38]. (A) An optical microscope image of a typical organsurface PVS [37,38]. (B) A schematic diagram of a Bonghan duct (BHD). Inside a single BHD, there are multiple Bonghan ductules (BH ductules) and the nuclei of their endothelial cells have a long oval shape [4]. In the circle, a phase contrast microscope image of the side view of the ductule bundle (red dashed rectangle) clearly shows the rod-shaped nuclei [3]. (C) A high-resolution, optical microscope image of a primo node (PN), harvested from the surface of a small intestine. Sub-primo vessels entering into a PN form small chambers/sinusoids (white stars) [11,37]. (D) Magnified view of cross section along the short axis of a sinusoid (in the direction of white dashed arrow in the green, dashed circle in (C). Many sanal-like entities (diamond green arrowheads) and small cell-like structures (arrows) are packed in the sinusoids [11,37].
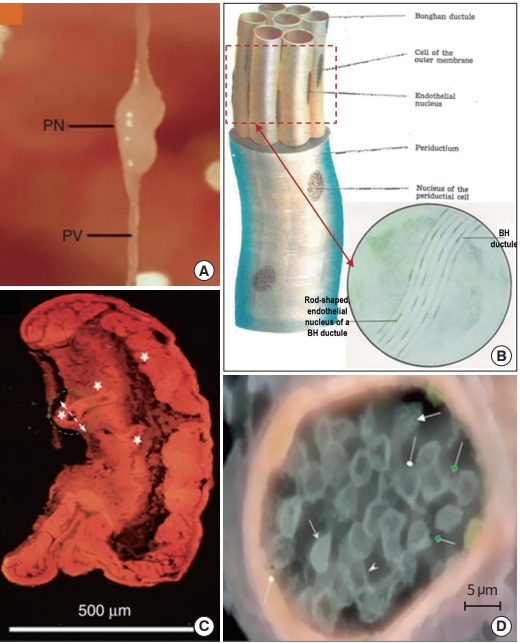
Fig. 8.
Images of sanals. (A) Electron microscopy image of Bonghan sanals, imaged by Bong Han Kim [4]. In the center of an individual sanal, there is a dark substance, which Kim called sanalosome and claimed to be a chromosome. (B) An optical microscopy image of sanals in a liquid medium. Each sanal looks like a micro-sized, transparent bubble containing a particle, which was confirmed to be DNA [37]. BSM, Bonghan sanal membrane; BSS, Bonghan sanalosome; BSP, Bonghan sanaloplasm.
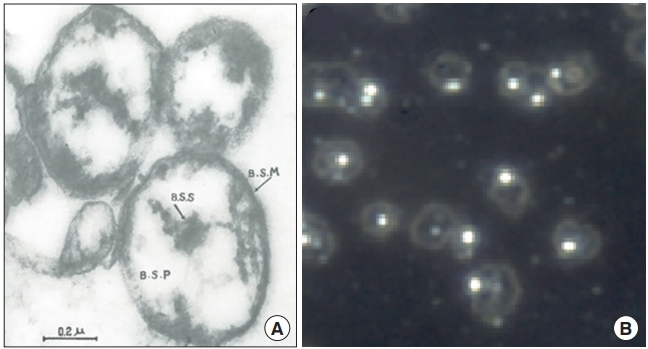
Fig. 9.
Bonghan Kim’s schematic diagram of the Bonghan sanal-cell cycle [4]. DNA in the nucleus of a cell gets dissected into individual chromosomes and these chromosomes are encapsulated to form sanals (sanalization; red line). These sanals move into the cytoplasm as the nuclear membrane gets disintegrated. As the cell membrane becomes loosened these sanals move out of the cell. The sanals out of the cells are transported to Bonghan corpuscles (BHCs) via Bonghan ducts and in the BHCs, sanals form new cells (cellation; blue line). This combined sanalization & cellation cycle is the ‘Bonghan sanal-cell cycle’ [4].

Fig. 10.
In vitro sanalization process with changes in time [4]. (A) (1-3) DNA is disintegrated into chromosomes, these chromosomes are encapsulated to form sanals, and sanals are released to cytoplasm as the nuclear membrane falls apart; (4, 5) The sanals escape from the cell as the cell membrane breaks up; (A*) The arrow points the sanals escaping from the loosened cell membrane in the animal tissue [4]. (B) An image of on-going, in vitro sanalization process. Adapted from unpublished data by the Vodyanoy team, with permission.
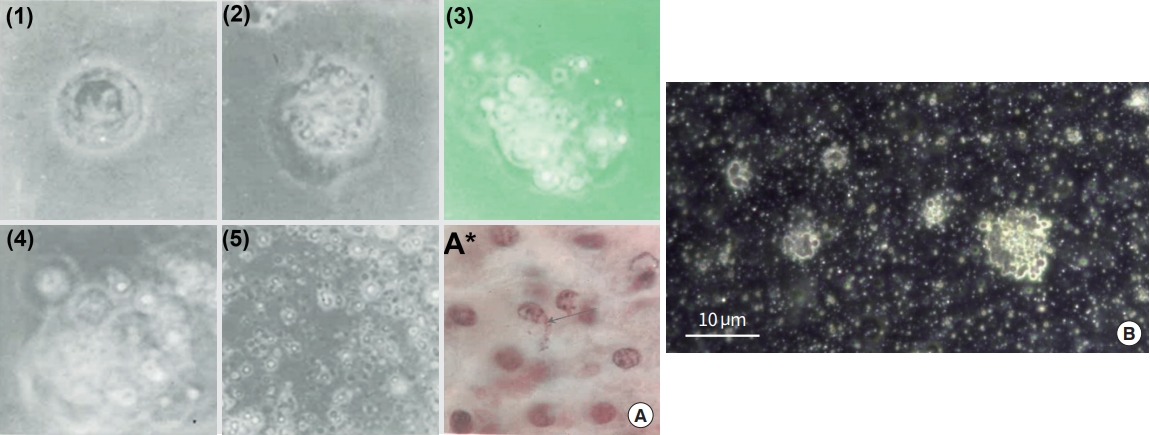
Fig. 11.
In vitro cellation processes [5,37]. (A) (1-3) Sanals appear to multiply; (4-6) These multiple sanals fuse together to form a nucleus-like structure; (7) The fused sanals forms a cell-like structure with small amount of cytoplasm; (8, 9) A cell with full cytoplasm is formed. (B) Merging of sanals in a liquid medium. (1, 2) When sanals are placed in a liquid medium they show incessant movements and start to merge. After a while they form spheroids and these spheroids grow in size; (3, 4) Once the spheroid reaches a certain size they are confined inside a fuzzy, membrane-like boundary. Soon the membrane-like structure becomes more defined, and inner materials start to show more cell-like features [37].
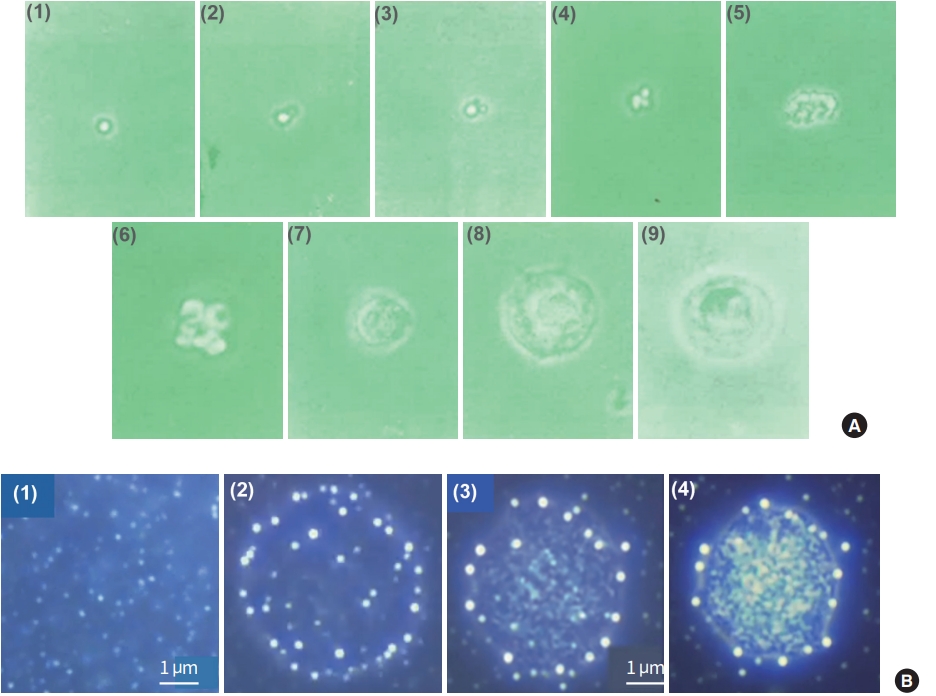
Fig. 12.
Primo vascular system (PVS) inside bone marrow [43]. (A) PVS being extracted from bone marrow of a rat model. (B) Expression of various biomarkers inside the sinusoid of a primo node (PN), extracted from bone marrow. This particular panel is one of several images that the Vodyanoy team produced for the biomarker expression of cells inside sinusoids. This panel shows five different biomarker expressions, including stem cell biomarkers, of cell-like bodies in a sinusoid of a PN inside bone marrow. The last image is the one without biomarker treatment. A hole that was previously occupied by the cell-like body next to it can be seen here (bars: 10 µm). PV, primo vessel; SOX2, SRY-box transcription factor 2; OCT4, octamer-binding transcription factor 4.

Fig. 13.
Optical images of primo vascular system (PVS) on human-to-nude mouse-xenografts. PVS on the xenografts of (A) lung cancer (NCI-H460); arrows, primo vessel (PV); arrowhead, tumor nodule [45]; (B) ovarian cancer (SK-OV-3): Quantum dot electroporated cancer cells are being transported from the primary tumor (P) to the secondary tumor via a lymphatic vessel (S1) and also to another secondary tumor via a PV [46]; (C) melanoma (B16BL6): white arrows, primo nodes (PNs); black dotted arrow, PV; black solid arrow, blood vessel [49]; (D) gastric cancer (MKN12): a translucent PV starting from the primary tumor is connected to the secondary tumor [49]; (E) leukemia (U937) (Adapted from Islam et al. [51], with permission from Academic Press Inc.); (F) breast cancer (MDA-MB231): white arrows, PNs; black dotted arrows, PVs [49]. Trypan blue was used as contrast agent, except (B) and (D). LV, lymphatic vessel; BV, blood vessel.
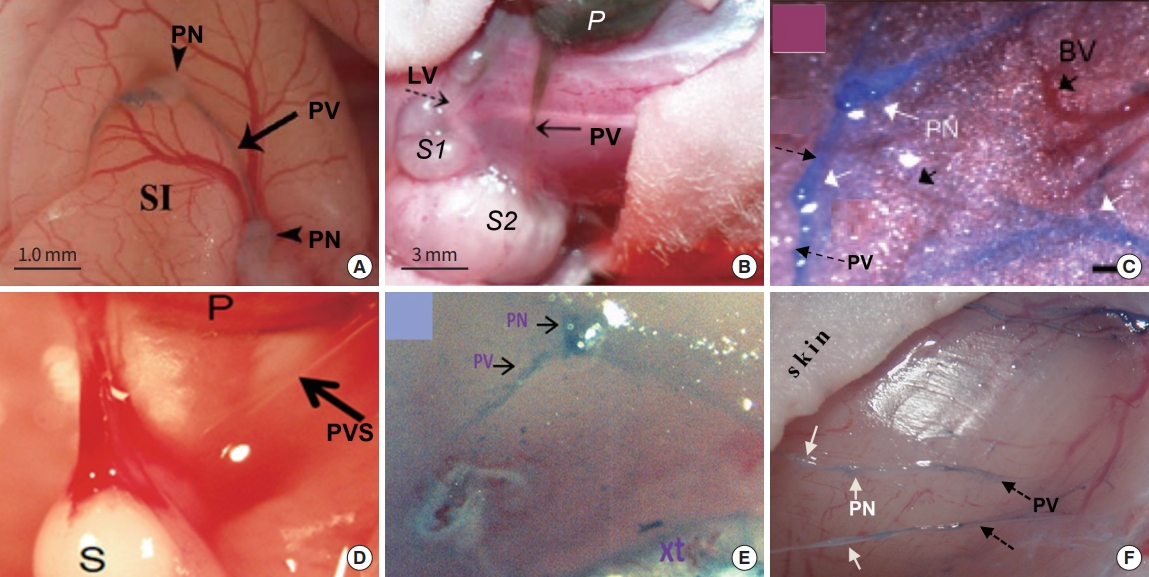
Table 1.
Terms for acupuncture meridian, Bonghan (Duct) system, and primo vascular system
REFERENCES
1. Kang KA. Historical observations on the half-century freeze in research between the Bonghan system and the primo vascular system. J Acupunct Meridian Stud 2013;6:285–92.


2. Kim BH. Great discovery in biology and medicine: substance of Kyungrak Pyongyang (KP): Foreign Languages Publishing House; 1962 Available from: www.ispvs.org.
3. Kim BH. On the Kyungrak system Pyongyang (KP): The Kyungrak Institute; 1964 Available from: www.ispvs.org.
4. Kim BH. Kyungrak system and theory of sanal. Proceedings of the Academic of Kyungrak of DPRK; 1965; Pyongyang, KP. Pyongyang (KP): Medical Science Press; 1965. Available from: www.ispvs.org.
5. Kim BH; on behalf of Kyungrak Institute of DPRK. [Bonghan sanal-cell cycle of blood cells]. J Acad Med Sci 1965;12:1. –7. Korean.
6. Kang KA. English translation of Bonghan Kim’s fifth report: Bonghan sanal-cell cycle of blood cells. J Acupunct Meridian Stud 2016;9:335–42.

7. Fujiwara S, Yu SB. ‘Bonghan theory’: morphological studies. Igakuno Ayumi 1967;60:567. –77. Japanese.
8. Soh KS, Kang KA, Ryu YH. 50 Years of Bong-Han theory and 10 years of primo vascular system. Evid Based Complement Alternat Med 2013;2013:587827.



9. Soh KS, Kang KA, Harrison DK. The primo vascular system: its role in cancer and regeneration. New York (NY): Springer; 2012.
10. Kim BH. Developmental and comparative biological study of primo vascular system: translation and edition with figures by Kwang-Sup Soh. J Acupunct Meridian Stud 2012;5:248–55.

11. Vodyanoy V, Pustovyy O, Globa L, Sorokulova I. Evaluation of a new vasculature by high resolution light microscopy: primo vessel and node. arXiv 2017;Jun 1 [Priprint]. https://doi.org/10.48550/arXiv.1608.04276.
12. Ogay V, Bae KH, Kim KW, Soh KS. Comparison of the characteristic features of Bonghan ducts, blood and lymphatic capillaries. J Acupunct Meridian Stud 2009;2:107–17.


13. Lim CJ, Lee SY, Ryu PD. Identification of primo-vascular system in abdominal subcutaneous tissue layer of rats. Evid Based Complement Alternat Med 2015;2015:751937.



14. Yoo JS, Kim MS, Ogay V, Soh KS. In vivo visualization of Bonghan ducts inside blood vessels of mice by using an Alcian blue staining method. Indian J Exp Biol 2008;46:336–9.

15. Lee C, Seol SK, Lee BC, Hong YK, Je JH, Soh KS. Alcian blue staining method to visualize Bonghan threads inside large caliber lymphatic vessels and X-ray microtomography to reveal their microchannels. Lymphat Res Biol 2006;4:181–90.


16. Johng HM, Yoo JS, Yoon TJ, Shin HS, Lee BC, Lee C, et al. Use of magnetic nanoparticles to visualize threadlike structures inside lymphatic vessels of rats. Evid Based Complement Alternat Med 2007;4:77–82.


17. Lee BC, Soh KS. Contrast-enhancing optical method to observe a Bonghan duct floating inside a lymph vessel of a rabbit. Lymphology 2008;41:178–85.

18. Lee SH, Bae KH, Kim GO, Nam MH, Choi YB, Kwon HM, et al. Primo vascular system in the lymph vessel from the inguinal to the axillary nodes. Evid Based Complement Alternat Med 2013;2013:472704.



19. Carlson E, Perez-Abadia G, Adams S, Zhang JZ, Kang KA, Maldonado C. A novel technique for visualizing the intralymphatic primo vascular system by using hollow gold nanospheres. J Acupunct Meridian Stud 2015;8:294–300.


20. Kang KA, Maldonado C, Vodyanoy V. Technical challenges in current primo vascular system research and potential solutions. J Acupunct Meridian Stud 2016;9:297–306.


21. Jiang X, Lee BC, Choi C, Baik KY, Soh KS, Kim HK, et al. Tubular substructure of intravascular thread-like structures from rats and rabbits. J Korean Phys Soc 2004;44:1602–4.
22. Baik KY, Lee JW, Lee BC, Johng HM, Nam TJ, Sung B, et al. Acupuncture meridian and intravascular Bonghan duct. Key Eng Mater 2005 277–279. 125–9.

23. Shin HS, Johng HM, Lee BC, Cho SI, Soh KS, Baik KY, et al. Feulgen reaction study of novel threadlike structures (Bonghan ducts) on the surfaces of mammalian organs. Anat Rec B New Anat 2005;284:35–40.


24. Lee BC, Yoo JS, Park ES, Yoon YS, Shin HS, Soh KS. Histological features of Bonghan corpuscles on the surface of rabbit internal organs. J Int Soc life inf Sci 2005;23:95–9.
25. Lee BC, Yoo JS, Ogay V, Kim KW, Dobberstein H, Soh KS, et al. Electron microscopic study of novel threadlike structures on the surfaces of mammalian organs. Microsc Res Tech 2007;70:34–43.


26. Sung B, Kim MS, Lee BC, Yoo JS, Lee SH, Kim YJ, et al. Measurement of flow speed in the channels of novel threadlike structures on the surfaces of mammalian organs. Naturwissenschaften 2008;95:117–24.


27. Lim CJ, Shen Y, Lee SY, Ryu PD. Potential erythropoiesis in the primo-vascular system in heart failure. Adv Exp Med Biol 2017;977:409–15.


28. Shen Y, Lim CJ, Lee SY, Ryu PD. Acute anemia induces erythropoiesis in rat organ surface primo-vascular tissue. Adv Exp Med Biol 2020;1232:385–392.


29. Lee BC, Kim S, Soh KS. Novel anatomic structures in the brain and spinal cord of rabbit that may belong to the Bonghan system of potential acupuncture meridians. J Acupunct Meridian Stud 2008;1:29–35.


30. Dai J, Lee BC, An P, Su Z, Qu R, Eom KH, et al. In situ staining of the primo vascular system in the ventricles and subarachnoid space of the brain by trypan blue injection into the lateral ventricle. Neural Regen Res 2011;6:2171–5.
31. Nam MH, Lim J, Choi SH, Kim S, Soh KS. A primo vascular system underneath the superior sagittal sinus in the brain of a rabbit. J Acupunct Meridian Stud 2012;5:210–7.


32. Lee HS, Kang DI, Yoon SZ, Ryu YH, Lee I, Kim HG, et al. Evidence for novel age-dependent network structures as a putative primo vascular network in the dura mater of the rat brain. Neural Regen Res 2015;10:1101–6.



33. Lee BC. Kim HB, Sung B, Kim KW, Sohn J, Son, B, et al. Structure of the sinus in the primo vessel inside the bovine cardiac chambers. In: Soh KS, Kang KA, Harrison DK, editors. The primo vascular system: its role in cancer and regeneration. New York (NY): Springer; 2012. p. 57-62.
34. Lee BC, Kim KW, Soh KS. Visualizing the network of Bonghan ducts in the omentum and peritoneum by using Trypan blue. J Acupunct Meridian Stud 2009;2:66–70.


35. Lee BS, Lee BC, Park JE, Choi HK, Choi SJ, Soh KS. Primo vascular system in human umbilical cord and placenta. J Acupunct Meridian Stud 2014;7:291–7.


36. Lee SY, Lee BC, Soh KS, Jhon G. Development of the putative primo vascular system before the formation of vitelline vessels in chick embryos. In: Soh KS, Kang KA, Harrison DK, editors. The primo vascular system: its role in cancer and regeneration. New York (NY): Springer; 2012. p. 77-82.
37. Kang KA, Pustovyy O, Globa L, Sorokulova I, Vodyanoy V. Sanal-cell cycle and primo vascular system: regeneration via Sanals. Adv Exp Med Biol 2018;1072:413–8.


38. Ogay V, Soh KS. Identification and characterization of small stem-like cells in the primo vascular system of adult animals. In: Soh KS, Kang KA, Harrison DK, editors. The primo vascular system: its role in cancer and regeneration. New York (NY): Springer; 2012. p. 149-55.
39. Kim MS, Oh SW, Lim JH, Han SW. Phase contrast X-ray microscopy study of rabbit primo vessels. Appl Phys Lett 2010;97:213703.

40. Kwon BS, Ha CM, Yu S, Lee BC, Ro JY, Hwang S. Microscopic nodes and ducts inside lymphatics and on the surface of internal organs are rich in granulocytes and secretory granules. Cytokine 2012;60:587–92.


41. Lee SJ, Park SH, Kim YI, Hwang S, Kwon PM, Han IS, et al. Adult stem cells from the hyaluronic acid-rich node and duct system differentiate into neuronal cells and repair brain injury. Stem Cells Dev 2014;23:2831–40.



42. Kucia M, Reca R, Campbell FR, Zuba-Surma E, Majka M, Ratajczak J, et al. A population of very small embryonic-like (VSEL) CXCR4(+)SSEA-1(+)Oct-4+ stem cells identified in adult bone marrow. Leukemia 2006;20:857–69.


43. Vodyanoy V, Pustovyy O, Globa L, Kulesza RJ Jr, Sorokulova I. Hemmule: a novel structure with the properties of the stem cell niche. Int J Mol Sci 2020;21:539.



44. Vodyanoy V, Pustovyy O, Globa L. Primo vascular node in the bone marrow and longevity. J Acupunct Meridian Stud 2022;15:12–24.


45. Hwang S, Lee SJ, Park SH, Chitteti BR, Srour EF, Cooper S, et al. Nonmarrow hematopoiesis occurs in a hyaluronic-acid-rich node and duct system in mice. Stem Cells Dev 2014;23:2661–71.



46. Yoo JS, Ayati MH, Kim HB, Zhang WB, Soh KS. Characterization of the primo-vascular system in the abdominal cavity of lung cancer mouse model and its differences from the lymphatic system. PLoS One 2010;5:e9940.



47. Yoo JS, Kim HB, Won N, Bang J, Kim S, Ahn S, et al. Evidence for an additional metastatic route: in vivo imaging of cancer cells in the primo-vascular system around tumors and organs. Mol Imaging Biol 2011;13:471–80.


48. Heo C, Hong MY, Jo A, Lee YH, Suh M. Study of the primo vascular system utilizing a melanoma tumor model in a green fluorescence protein expressing mouse. J Acupunct Meridian Stud 2011;4:198–202.


49. Walter A, Liu Y, Sudlow G, Lee J, Yoo JS, Lee BC, et al. Identification of primo vascular system in murine tumors and viscera. In: Soh KS, Kang KA, Harrison DK, editors. The primo vascular system: its role in cancer and regeneration. New York (NY): Springer; 2012. p. 179-83.
50. Kang KA, Maldonado C, Perez-Aradia G, An P, Soh KS. Primo vascular system and its potential role in cancer metastasis. Adv Exp Med Biol 2013;789:289–96.


51. Islam MA, Thomas SD, Sedoris KJ, Slone SP, Alatassi H, Miller DM. Tumor-associated primo vascular system is derived from xenograft, not host. Exp Mol Pathol 2013;94:84–90.









