Systemic vasculopathy associated with an RNF213 p.Arg4810Lys variant in moyamoya disease: A review
Article information
Abstract
Moyamoya disease (MMD) is a steno-occlusive disease of the distal cerebral arteries. Ring finger protein 213 (RNF213) p.Arg4810Lys is a susceptibility gene of MMD for which related vasculopathies are not well characterized. Heterozygous patients were mostly asymptomatic or exhibited isolated MMD. Homozygous patients showed a very unique pattern of diffuse narrowing of the entire aorta along with stenosis of the splanchnic, renal, coronary, iliofemoral, and/or peripheral pulmonary arteries, regardless of presence or absence of MMD. RNF213 p.Arg4810Lys is associated with high penetrance of systemic vasculopathy in homozygous patients and low penetrance of intracranial stenosis, i.e., MMD, in heterozygous patients, which suggests a gene-dosage effect.
INTRODUCTION
Moyamoya disease (MMD) is an idiopathic progressive angiopathy affecting the terminal portions of the internal carotid arteries. The familial occurrence pattern of the disease implies the existence of genetic risk factors. The mode of inheritance of familial MMD is autosomal dominant with incomplete penetrance. Although several monogenic diseases are complicated by moyamoya angiopathy, the ring finger protein 213 gene (RNF213) was identified as a susceptibility gene for MMD[1,2]. This gene encodes a relatively large protein with dual AAA+ ATPase and E3 ligase activities [3]. In vitro and in vivo experiments indicate that RNF213 is related to angiogenesis and vascular inflammation, but the physiologic functions of RNF213 remain incompletely known [3]. The RNF213 p.Arg4810Lys variantis an Asian founder mutation, and the prevalence of this variant has been reported to be as high as 2.5% in East Asians [4-6]. The variant is not found in western populations. Because the total number of carriers of the RNF213 p.Arg 4810Lys variant in Asian countries is estimated to be 15 million, the impact of this allele on cardiovascular health is substantial [3]. In Korea, estimated numbers of heterozygotes and homozygotes are 1.25 million and 5,000 individuals, respectively, of a 50 million person population [6].
The prevalence of heterozygotes and homozygotes of the RNF213 p.Arg4810Lys variant is reported to account for 70%–80% and 7%–8%,respectively, of all MMD patients [3]. Homozygotes are associated with earlier onset and more severe forms of MMD[7,8]. The penetrance rate of MMD in individuals heterozygous for the RNF213 p.Arg4810Lys variantis as low as one per 150–300 [9-11], and the penetrance rate of MMD for homozygotes is calculated to be greater than 78% [7]. Extracranial involvement of MMD has been described among patients with coronary artery [12,13], pulmonary artery [14], and renal artery stenosis, including 7.9% of pediatric MMD patients [15], although there was no genetic information provided in those studies. A recent suggestion is that the RNF213 p.Arg4810Lys variant causes classical MMD when heterozygous, but that the same variant results in MMD and systemic vascular diseases when homozygous in a gene dosage-dependent manner [14,16]. In this review, extracranial manifestations of RNF213 p.Arg4810Lys-related systemic vasculopathies are described.
AORTA
Homozygosity of RNF213 p.Arg4810Lys can affect the entire vascular system, including the aorta. Involvement of the aorta shows a very unique pattern, i.e., diffuse narrowing of the entire aorta, with the most prominent narrowing observed in the infra-renal abdominal aorta. This type of aortic involvement was not previously reported in patients with MMD or other vasculopathies. Aortic narrowing or stenosis can occur in Williams syndrome, coarctation ofthe aorta, midaortic syndrome, and various forms of aortitis such as Takayasu arteritis. Patients with Williams syndrome have supravalvular aortic stenosis, while the remaining portion of the aorta shows normal contours. In patients with coarctation of the aorta or midaortic syndrome, aortic involvement is focal or segmental; in those with aortitis, the aortic involvement site shows diffuse wall thickening with enhancement on computed tomography (CT) angiography. RNF213 p.Arg4810Lys homozygous patients did not show any signs of vascular inflammation. The narrowing of the aorta continued to the level of the iliofemoral artery. Heterozygosity of RNF213 p.Arg4810Lys also can affect the aorta to a mild degree. Fig. 1 shows the gene dosage effect of aortic involvement. The homozygous patient’s aorta is narrowed in its entire length, and the diameter is mostly reduced in the infrarenal abdominal aorta. However, aortic narrowing of heterozygous patients is limited to the infrarenal portion to a mild degree. Prevalence of aortic narrowing of RNF213 p.Arg4810Lys-related vasculopathy is unknown, but homozygous patients show more severe and more prevalent aortic involvement than heterozygotes.
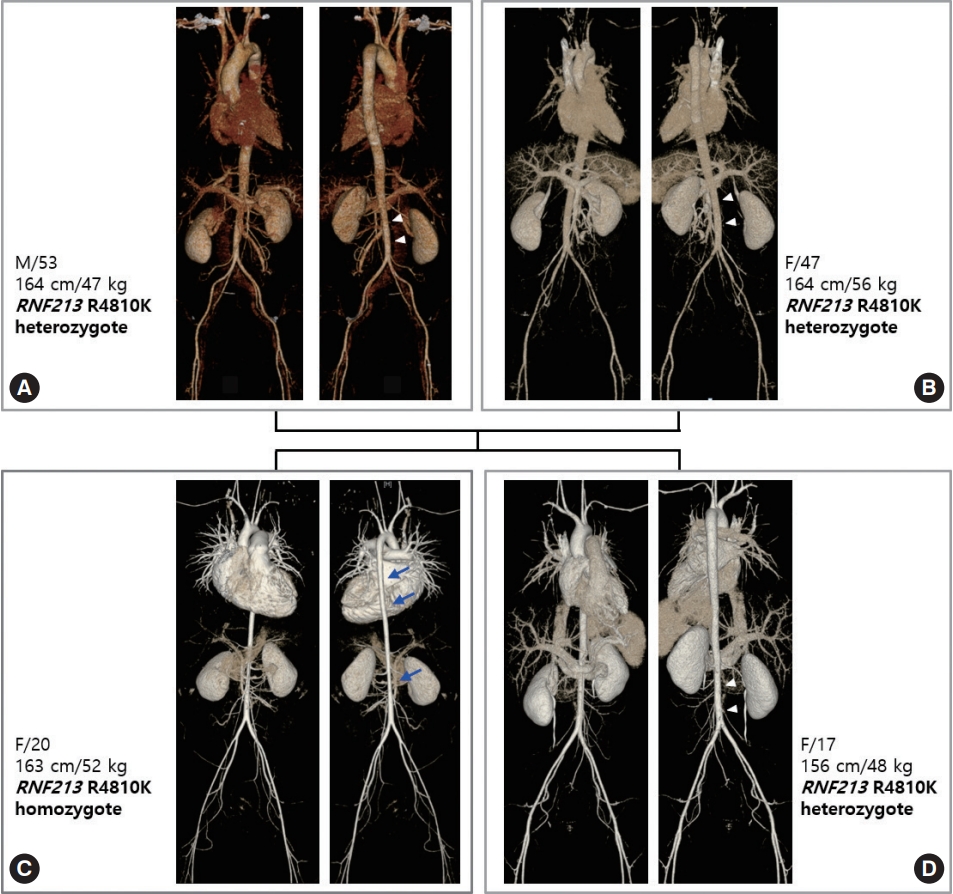
Vascular family tree of ring finger protein 213 (RNF213) p.Arg4810Lys-related systemic vasculopathy. (A) Father, (B) mother, (C) index case, (D) younger sister of index case. The index patient, a 20-year-old female, is a homozygote and with heterozygote family members. The homozygous index patient has diffuse narrowing of the aorta (arrows), most prominent in the descending thoracic aorta and infra-renal abdominal aorta. Vascular narrowing continues to the level of the iliofemoral arteries. Other heterozygous family members show milder narrowing of the infra-renal abdominal aorta (arrowheads). Although the sibling of the index case has a smaller body habitus than her older sister, her aorta is larger.
VISCERAL ARTERIES
Homozygosity of RNF213 p.Arg4810Lys affects visceral arteries such as the superior mesenteric, celiac, and renal arteries (Fig. 2). The involvement is characterized by ostial stenosis. The stenotic superior mesenteric and celiac arteries do not develop mesenteric ischemia or abdominal angina because, unlike atherosclerotic occlusion, these patients have patent inferior mesenteric arteries that provide collateral circulation. Aside from asymptomatic splanchnic arterial stenosis, renal involvement of homozygosity of RNF213 p.Arg4810Lys could induce renal artery stenosis with resultant renovascular hypertension. RNF213 p.Arg4810Lys-related systemic vasculopathy should be included in the differential diagnosis of renovascular hypertension, especially in younger patients. Occasionally, in young renovascular patients, a genetic testinitially reveals RNF213 p.Arg4810Lys homozygosity; and the following systemic imaging study discloses asymptomatic brain involvement, i.e., MMD. The renal involvement of RNF213 p.Arg4810Lys-related systemic vasculopathy shows different patterns of stenosis compared to those of fibromuscular dysplasia (FMD). Imaging studies of FMD demonstrate several patterns: (1) typical string of beads; (2) focal stenosis of proximal renal artery; and (3) diffuse stenosis of middle to distal renal artery. However, RNF213 p.Arg4810Lys homozygous patients show diffuse ostial stenosis as in Fig. 2. Rarely, heterozygous patients have diffuse ostial renal artery stenosis that is similar to homozygous renal artery stenosis.

Visceral involvement of ring finger protein 213 (RNF213) p.Arg4810Lys homozygosity. (A) Computed tomography (CT) shows severe ostial stenosis (arrow) of the celiac trunk in a 20-year-old female patient. (B) CT shows severe ostial stenosis (arrow) of the superior mesenteric artery in a 13-year-old male patient. (C) Angiogram shows diffuse ostial stenosis (arrows) of both renal arteries in a 16-year-old male patient.
PULMONARY ARTERY
Previously, we reported pulmonary involvement of five patients with RNF213 p.Arg4810Lys homozygosity [16]. Pulmonary angiograms showed a unique “string of beads” pattern and/or diffuse stenosis of peripheral pulmonary arteries (Fig. 3). Perfusion scans of the lung revealed multiple segmental to subsegmental defects that could not be differentiated from lung scan findings of patients with chronic thromboembolic pulmonary hypertension (CTEPH). All of these patients had history of treatment for a presumed diagnosis of CTEPH, pulmonary vasculitis, or idiopathic pulmonary arterial hypertension. Even though these patients showed severe pulmonary hypertension and no response to medical therapy, their course was relatively benign except for two patients who died. One of these deaths was presumed to be a sudden cardiac-related death at after 11 years follow-up, and the other patient died due to sepsis. Notably, three patients had MMD and two patients did not have MMD. The three MMD patients had multiple stenosis of extracranial arteries other than the pulmonary artery. Homozygotes for the MMD susceptibility gene RNF213 p.Arg4810Lys experienced pulmonary hypertension from peripheral pulmonary artery stenosis (PPAS) in segmental or subsegmental arteries in adulthood, with multiple extracranial vasculopathies. RNF213 variant-related vasculopathy should be categorized as an entity of adulthood-onset PPAS regardless of presence or absence of MMD.
Pulmonary involvement of ring finger protein 213 (RNF213) p.Arg4810Lys homozygosity. Pulmonary angiogram shows multifocal “string of beads” stenosis (A, arrowheads) of segmental to subsegmental pulmonary arteries in a 26-year-old male patient and diffuse rarefaction (B, arrows) of distal pulmonary arteries in a 23-year-old female patient.
CORONARY ARTERY
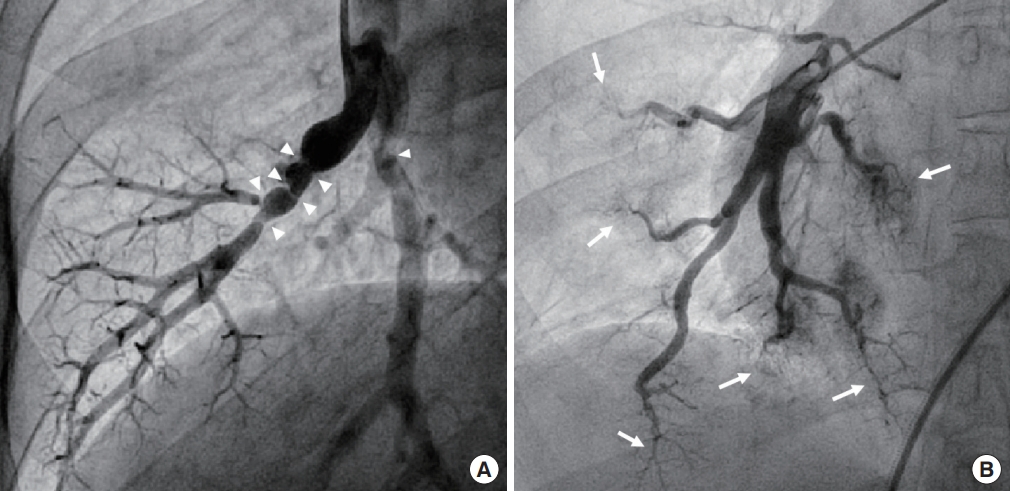
Colleagues of our institute reported that 4.6% of MMD patients had symptomatic coronary heart disease [12]. The median age of these patients was 44 years, and the median Framingham score at diagnosis of MMD was low (<1%). These colleagues concluded that, considering the relatively young age of onset and low risk of coronary artery disease (CAD), CAD might be a clinically relevant, systemic manifestation of MMD. Homozygous patients can have several patterns of coronary involvement: (1) coronary ostial stenosis; (2) diffuse spastic coronary arteries; and/or(3) variant angina (vasospasm) (Fig. 4). When cardiologists encounter ostial lesions with diffusely spastic coronary arteries, especially in younger patients, RNF213 p.Arg4810Lys-related vasculopathy should be suspected.

Coronary involvement of ring finger protein 213 (RNF213) p.Arg4810Lys homozygosity. Coronary angiogram shows severe stenosis (arrow) of the left main coronary artery (A) and diffuse spasticity of the entire coronary arteries (A, B) in a 20-year-old male patient. Coronary angiogram shows mild stenosis (C, arrow) and severe spasm (D, arrow) after ergonovine test of the proximal left anterior descending coronary artery in a 19-year-old female patient. Coronary arteries are diffusely spastic in a basal angiogram before ergonovine injection (C).
TREATMENT OF SYSTEMIC VASCULOPATHY
The best treatment plan for RNF213 p.Arg4810Lys homozygosity-related systemic vasculopathy is unknown but will be complex. As described, splanchnic stenosis (superior mesenteric and/or celiac artery) does not require intervention because these patients are asymptomatic. Renal artery stenosis and PPAS cause renovascular and pulmonary hypertension, respectively. Coronary involvement induces angina. When symptomatic, percutaneous angioplasty can be applied in some segmental arteries. However, vasculopathy in MMD involves intimal hyperplasia of smooth muscle cells and possibly results in immediate elastic recoil or progressive restenosis. These were seen in MMD patients undergoing angioplasty of cerebral arteries [17]. Therefore, percutaneous angioplasty should be performed with caution in these patients. In cases of diffuse stenosis, the possibility of rupture of the vessel wall during angioplasty should be considered. Extreme care must be exercised when conducting percutaneous angioplasty with or without stenting in these patients. When stenosis is resistant to percutaneous dilatation or restenosis occurs, surgical revascularization is the next treatment option for renal or coronary stenosis.
MMD had been regarded as a disease of neurologists or neurosurgeons. However,recent findings reveal that the gene dosage effect of RNF213 p.Arg4810Lys could induce systemic vasculopathies that other specialists such as cardiologists, nephrologists, pulmonologists, and cardiovascular surgeons concern. RNF213 p.Arg4810Lys-related systemic vasculopathy should be included in the differential diagnosis when clinicians encounter (1) diffuse narrowing of the aorta with especially prominent narrowing of the abdominal aorta;(2) diffuse ostial stenosis of the celiac, superior mesenteric, renal, and/or coronary arteries; (3) diffuse spasticity of the coronary artery; and/or (4) stenosis of the segmental/subsegmental pulmonary artery, especially in young patients.
CONCLUSION
In summary, heterozygous patients of RNF213 p.Arg4810Lys are mostly asymptomatic or exhibit isolated MMD. Homozygous patients show a very unique pattern of diffuse narrowing of the entire aorta along with stenosis of the splanchnic, renal, coronary, iliofemoral, and/or peripheral pulmonary arteries,regardless of presence or absence of MMD.
Notes
No potential conflict of interest relevant to this article was reported.
AUTHOR CONTRIBUTIONS
Conception or design: DKK.
Acquisition, analysis, or interpretation of data: DKK, SYJ, SAC, TKP.
Drafting the work or revising: DKK.
Final approval of the manuscript: DKK.
Acknowledgements
This study was supported by a grant from Samsung Medical Center (#OTX000011).
