Minimally invasive mediastinal staging of non-small cell lung cancer
Article information
Abstract
Endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA) is a minimally invasive procedure widely used for the diagnosis and staging of primary lung cancer. This review focuses on the role of EBUS-TBNA in minimally invasive mediastinal staging, restaging after induction therapy, and procedure-related issues. To better understand the role of EBUS-TBNA, one must consider issues of sedation and rapid onsite examination, sonographic features during the procedure, the number of aspirations per lymph node, and the thoroughness of the procedure. A literature review indicated that EBUS-TBNA showed equivalent or even superior performance to mediastinoscopy in the mediastinal staging of non-small cell lung cancer (NSCLC). Combining endoscopic ultrasound-guided fine needle aspiration (EUS-FNA) or the transesophageal approach using an EBUS bronchoscope (EUS-B-FNA) with EBUS-TBNA can provide additional diagnostic benefits. A recent guideline recommended endosonography over mediastinoscopy as the initial procedure for mediastinal nodal staging in patients with NSCLC with abnormal mediastinal and/or hilar lymph nodes on chest computed tomography (CT) or positron emission tomography/CT. The diagnostic sensitivity of EBUS-TBNA for restaging after induction therapy in patients with stage IIIA-N2 NSCLC was lower than that of initial staging. It appears reasonable to perform EBUS-TBNA first for initial mediastinal staging and reserve mediastinoscopy for restaging after induction therapy.
INTRODUCTION
Lung cancer is the leading cause of cancer-related death in both male and female patients [1]. Mediastinal nodal staging plays a very important role in the management of non-small cell lung cancer (NSCLC) because it can predict patient survival and allow for the planning of an appropriate treatment strategy. The International Association for the Study of Lung Cancer (IASLC) has updated the TNM (tumor, node, and metastasis) staging system, including the mediastinal nodal map guidelines [2,3]. Mediastinal nodal staging of primary lung cancer is characterized from N0 to N3 according to the metastatic nodal stations from the primary tumor site. Mediastinal nodal staging before surgery includes noninvasive modalities, such as chest computed tomography (CT) and positron emission tomography (PET)/CT, or invasive surgical methods represented by mediastinoscopy. Endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA) is a minimally invasive staging method and has had a major clinical impact on lung cancer staging since it was first reported in 2003 [4]. EBUS-TBNA has been used clinically for mediastinal nodal staging of NSCLC and restaging following induction treatment and for the diagnosis of benign or malignant mediastinal disease. This review focuses on the role of EBUS-TBNA in minimally invasive mediastinal staging, restaging after induction therapy, and procedure-related issues.
PRACTICAL ISSUES FOR ACHIEVING HIGH ACCURACY BY EBUS-TBNA
Sedation for EBUS-TBNA
EBUS-TBNA requires operator experience because the EBUS bronchoscope (EUS-B-FNA) must be inserted through the vocal cord with a 35° forward oblique view. Furthermore, EBUS-TBNA requires more time to perform than flexible bronchoscopy. Thus, sedation is important for yielding the best diagnostic results with optimal patient comfort and minimizing related complications. In a randomized prospective trial of 149 patients, Casal et al. [5] reported no difference in diagnostic yield between a deep sedation group and a moderate sedation group. Another study by Dal et al. [6] also assessed patient comfort and satisfaction by sedation type (divided by ketamine-midazolam and ketamine-propofol combinations) and found no advantages in the deep sedation group. Therefore, moderate sedation appears to be sufficient for EBUS-TBNA.
Training and learning curve
After the EUS-B-FNA is inserted through the vocal cords, endosonography is performed to search for the target lymph nodes and a needle set is inserted into the working channel to obtain aspirates and core tissues from the target lesions. The different view and unique technique make it difficult for inexperienced users to obtain good-quality samples. Stather et al. [7] reported evidence of the effectiveness of an EBUS simulator compared to conventional training methods. A multicenter cohort study showed that fellows in pulmonary medicine could obtain adequate tissue after performing an average of 13 procedures [8]. However, there is significant variation in the learning curve in training fellows because about 33% did not achieve an expert level of technique during this training program [9]. In a randomized controlled trial, Konge et al. [10] suggested that virtual reality simulator training was more effective than traditional apprenticeship training.
Sonographic features and elastography
Another important issue is understanding the ultrasound features detected by EBUS. It is essential to discriminate the characteristics of benign and malignant lymph nodes on ultrasound images. Fig. 1 shows sonographic features for predicting metastatic lymph nodes, including a rounded shape, distinct margins, heterogeneous echogenicity, the presence of a coagulation necrosis sign, and a central hilar structure, as suggested by Fujiwara et al. [11]. However, in regions where tuberculous or anthracotic lymph nodes are prevalent, these criteria cannot clearly discriminate between benign and malignant lesions because benign lesions also show a heterogeneous echotexture and coagulation necrosis sign similar to those of metastatic lymph nodes (Fig. 2) [12,13]. Despite their imperfect ability to predict metastasis, sonographic features can help the bronchoscopist to select suspicious targets of most metastatic lymph nodes, particularly when the patient’s condition does not allow for a full evaluation of lymph nodes. In previous studies, power/color Doppler-mode vascular image patterns were also helpful in predicting malignancy [14,15]. The presence of a central intranodal blood vessel suggests that the node is benign, whereas the absence of a central intranodal vessel increases the likelihood of malignancy [14]. The presence or absence of a central intranodal blood vessel has good overall accuracy in predicting malignancy (87.4%) [14]. The blood flow from the bronchial artery toward the lymph node visualized as blue signals on EBUS color Doppler-mode images was also helpful in predicting malignancy, and the accuracy of predicting metastasis solely from a positive bronchial artery inflow sign was 80.3% [15].
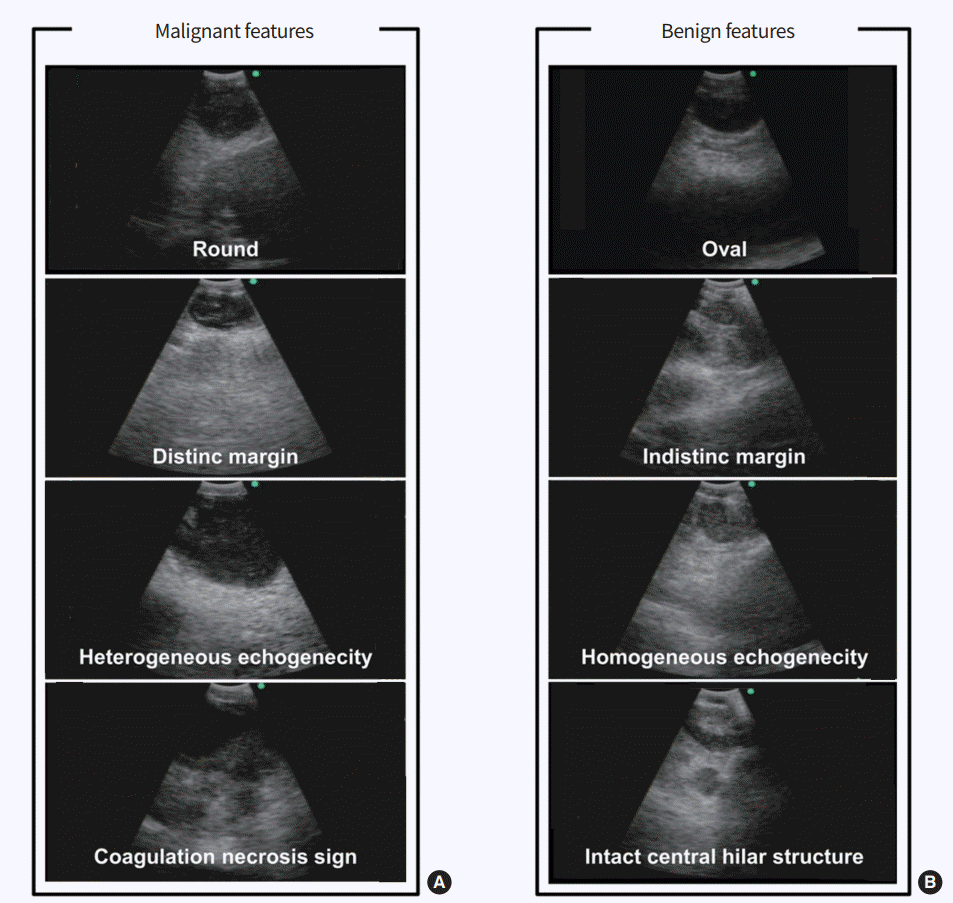
(A) Malignant features include round shape, distinct margin, heterogeneous echogenecity, and coagulation necrosis sign. (B) Benign features include oval shape, indistinct margin, homogeneous echogenecity, and intact hilar structure.
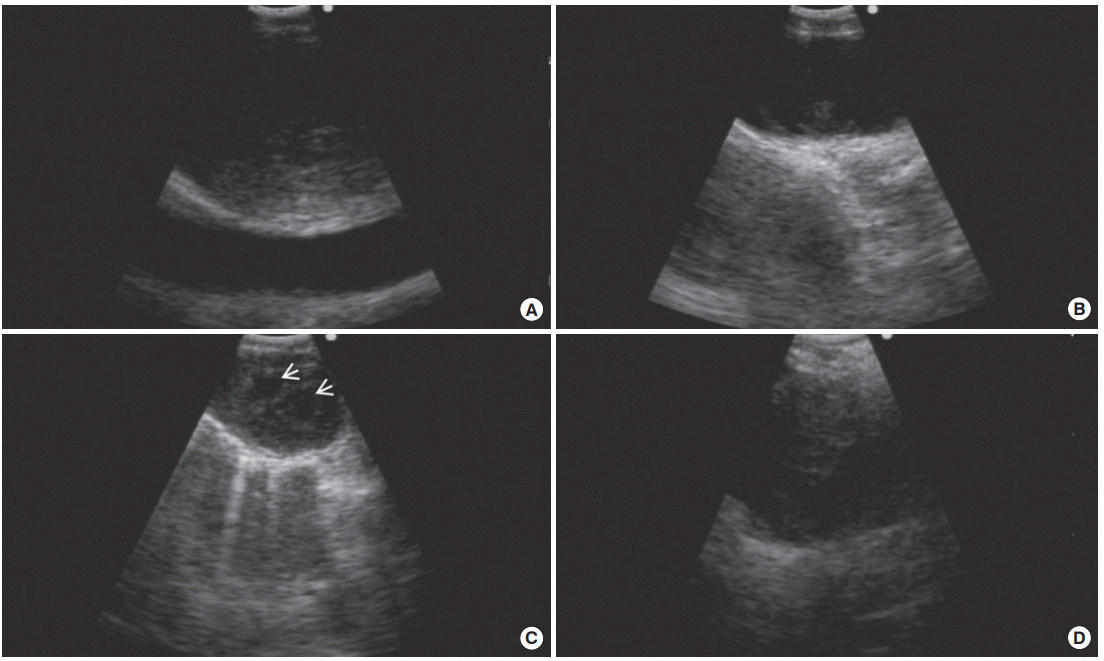
Similarity of endobronchial ultrasound features of tuberculous lymphadenopathy and anthracotic lymph nodes compared to malignant lymph nodes. (A) A sonographic image of a metastatic node showed heterogeneous echogenicity. (B) Endobronchial ultrasound revealed a coagulation necrosis sign in the metastatic lymph node. (C) A coagulation necrosis sign (arrows) was observed in tuberculous lymphadenopathy. Mycobacterium tuberculosis was cultured in the lymph node aspirate. (D) An ultrasound image demonstrated heterogeneous echogenicity in the anthracotic lymph node.
Elastography has also been used to predict and localize metastatic lymph nodes during EBUS. Neoplastic tissue is usually stiffer than normal tissue, and elastography can generate images of stiffer tissue in metastatic lymph nodes. In a previous study, mean stiff area ratios were significantly greater for metastatic lymph nodes (0.478) than for benign lymph nodes (0.216; P= 0.0002), and the stiff area was histologically compatible with the metastatic distribution in surgically resected lymph nodes [16].
Aspiration of lymph nodes
The optimal number of aspirations to improve diagnostic yield is of considerable importance in mediastinal evaluation using EBUS-TBNA. In a prospective study that compared EBUS-TBNA and mediastinoscopy for mediastinal staging, EBUS-TBNA sampled an average of three (one to six) nodal stations in a clinical setting [17]. Although two reports regarding conventional TBNA indicated that a maximum of seven aspirations was sufficient for optimal diagnostic yield [18,19], Lee et al. [20] suggested that optimal results could be obtained with three aspirations per lymph node station in EBUS-TBNA for mediastinal staging of potentially operable NSCLC when rapid onsite evaluation (ROSE) was not available. In this prospective study, three aspirations per node could reach a plateau of 100% adequacy and 95% sensitivity without additional yield after the fourth aspiration. When at least one tissue core specimen was obtained by the first or second aspiration, two aspirations per lymph node station were acceptable.
Thoroughness of the procedure and ROSE
The accuracy of mediastinal staging depends not only on which test is used but also on how the procedure is performed. Needle-based techniques, such as EBUS-TBNA and endoscopic ultrasound-guided fine needle aspiration (EUSFNA), can be divided into four categories according to sampled nodal stations, number of aspirations per node, and performance of ROSE: complete, systemic, selective, and poor sampling [21]. Complete sampling involves the sampling of each visible node at each station (1, 2R, 2L, 3, 4R, 4L, 7, 8, and 5, 6 if left upper lobe tumor), ≥ 3 passes per node, or ROSE. Systemic sampling involves sampling nodes in each station (2R, 2L, 4R, 4L, 7, and 5, 6 if left upper lobe tumor) and ≥ 3 passes per node or ROSE. Selective sampling involves biopsying one or more stations that must include a node suspicious on imaging analysis or ≥ 1 cm on ultrasonography if present, or < 3 passes per node and no ROSE. Although complete sampling is ideal for mediastinal nodal staging, it has limitations in clinical practice during needle-based procedures because it prolongs the procedure time and may increase patient discomfort and procedure-related complications. However, to achieve optimal results, systemic nodal sampling should be performed using needle-based techniques [21].
MINIMALLY INVASIVE MEDIASTINAL STAGING: EBUS-TBNA, EUS-FNA, AND EUS-B-FNA
Mediastinal nodal staging before the treatment of lung cancer is clinically important, as it can guide the treatment strategy. Mediastinal nodal evaluation first involved noninvasive methods based on image analysis (such as chest CT and PET/CT), and then invasive methods (such as EBUS-TBNA, EUSFNA, or mediastinoscopy) were used to examine otherwise inaccessible tissues. Although chest CT and PET/CT are now widely used in evaluating lung cancer and provide nodal information for the mediastinum, treatment decisions should not be made based on imaging studies alone because sensitivity and specificity for mediastinal staging are approximately 55% and 81%, respectively, for chest CT and 62% and 90%, respectively, for PET/CT [22]. Therefore, imaging findings suggestive of metastasis should be confirmed pathologically before treatment decisions are made. Minimally invasive needle techniques, such as EBUS-TBNA and EUS-FNA, have increasingly been used to stage the mediastinum. In contrast to mediastinoscopy, which requires general anesthesia, EBUS-TBNA can be performed safely under conscious sedation using midazolam and fentanyl.
EBUS-TBNA alone
A convex EBUS-TBNA probe was first introduced in 2003 and showed sensitivity, specificity, and accuracy of 95.7%, 100%, and 97.1%, respectively [23]. It could distinguish benign from malignant lymph nodes with high diagnostic yields. A 2009 meta-analysis for staging of lung cancer that included a total 1,299 patients in 11 studies assessed the overall diagnostic accuracy of EBUS-TBNA and showed a pooled diagnostic sensitivity of 93% and pooled specificity of 100% [24]. These results suggest that mediastinal staging by EBUS-TBNA has better accuracy than chest CT and PET/CT scans. However, EBUS-TBNA could not evaluate stations 5, 6, 8, and 9 because of inaccessibility. These stations can be sampled by the transesophageal approach using EUS-FNA. Therefore, a combination of EBUS-TBNA and EUS-FNA is useful for complete and systematic mediastinal staging.
EUS-FNA alone
EUS-FNA is another minimally invasive method for mediastinal staging of NSCLC and was used prior to the introduction of EBUS-TBNA. Micames et al. [25] performed a meta-analysis of 18 eligible studies and reported pooled diagnostic sensitivity and specificity of EUS-FNA for NSCLC staging of 83% and 97%, respectively. The median prevalence of malignant lymph nodes in this analysis was 65%. They reported that the major limitation of this technique was an inability to access the 2R, 3, 4R, and 6 nodal stations, and they suggested using a combination of EBUS-TBNA and EUS-FNA to overcome this.
Combined EBUS-TBNA and EUS-FNA
EBUS-TBNA and EUS-FNA complement each other for nodal staging because nodal stations 2L, 4L, 5, 7, 8, and 9 can be reached by EUS-FNA and nodal stations 1R, 1L, 2R, 2L 3P, 4R, 4L, 7, 10, and 11 can be accessed by EBUS-TBNA. Combining the two techniques could lead to complete mediastinal staging because nearly all mediastinal stations, with the exception of stations 5 and 6, can be accessed by the two techniques. A procedure involving a combination of transbronchial and transesophageal approaches has increasingly been used, and there have been no reports of serious complications. In a meta-analysis of eight studies with 821 patients, Zhang et al. [26] reported that the combined technique was more sensitive than EBUS-TBNA or EUS-FNA alone, with pooled diagnostic sensitivity and specificity of 86% and 100%, respectively.
Combined EBUS-TBNA and EUS-B-FNA
Although combining EBUS-TBNA and EUS-FNA results in more accurate mediastinal nodal staging, it has several limitations in clinical practice. It requires expert endoscopists and equipment as well as additional costs and time for the evaluation. It would be ideal if EBUS-TBNA and EUS-FNA could be performed by the same operator. Hwangbo et al. [27] first reported the feasibility of the transesophageal approach using an EUS-B-FNA for lung masses and lymph nodes that were inaccessible by EBUS-TBNA and obtained additional diagnostic benefit. Lee et al. [28] also reported the additional value of EUS-B-FNA for patients with nodal stations inaccessible by EBUS-TBNA, and 13% of the patients examined by the combined approach were upstaged. A random effect meta-analysis showed that adding EUS-B-FNA to EBUS-TBNA increased sensitivity by 21% compared to EBUS-TBNA alone [29]. Another meta-analysis of 13 studies found additional value in the combination of EBUS-TBNA and EUS-(B)-FNA, with mean sensitivity of 86% for the combined approach [30]. There were no significant differences in mean sensitivity or negative predictive value between studies that used EBUS first or EUS first or between studies that used an EBUS-scope or a regular endoscope to perform EUS.
EBUS-TBNA/EUS-FNA vs. mediastinoscopy
Mediastinoscopy was the gold standard for invasive mediastinal staging prior to the introduction of the needle techniques. Mediastinoscopy requires general anesthesia and has morbidity and mortality rates of 2% and < 0.1%, respectively. It has limited accessibility and only allows for an examination of stations 2R, 2L, 4R, 4L, and 7. Four prospective studies were performed to compare the diagnostic performance of endosonography and mediastinoscopy (Table 1). Ernst et al. [31] performed a crossover trial comparing the diagnostic performance of EBUS-TBNA and cervical mediastinoscopy in patients with suspected NSCLC. EBUS-TBNA had a higher overall diagnostic yield (91%) compared to mediastinoscopy (78%) in per lymph node analysis. There was disagreement in the yield between the two procedures in the subcarinal lymph nodes (24%). In a prospective controlled trial by Yasufuku et al. [32] compared EBUS-TBNA to mediastinoscopy for mediastinal nodal staging of potentially resectable NSCLC; all patients underwent EBUS-TBNA followed by mediastinoscopy under general anesthesia. EBUS-TBNA and mediastinoscopy achieved similar results for mediastinal staging of NSCLC. Um et al. [17] conducted a prospective trial among patients with histologically proven NSCLC and suspected N1, N2, or N3 metastasis; each patient underwent EBUS-TBNA followed by mediastinoscopy. The diagnostic sensitivity of EBUS-TBNA was superior to that of mediastinoscopy (88.0% vs. 81.3%). EBUS-TBNA had higher diagnostic sensitivity, particularly in stations 4L (81.0% vs. 52.4%) and 7 (82.5% vs. 75.0%). Annema et al. [33] conducted a randomized controlled multicenter trial of 241 patients with resectable NSCLC to compare combined endosonography and mediastinoscopy. A combination of endosonography and surgical staging resulted in greater sensitivity for mediastinal nodal metastases (85% vs. 79%, respectively) and fewer unnecessary thoracotomies (7% vs. 18%, respectively) than surgical staging alone. Based on these prospective studies, recent guidelines published by ESGE/ERS/ESTS (European Society of Gastrointestinal Endoscopy/European Respiratory Society/European Society of Thoracic Surgeons) recommend endosonography over mediastinoscopy as the initial procedure for mediastinal nodal staging in patients with NSCLC with abnormal mediastinal and/or hilar nodes on chest CT or PET/CT [29]. However, subsequent mediastinoscopy is recommended when endosonography does not show metastasis.
Interpretation of negative results of endosonography
It is important to identify predictors of false-negative results on endosonography because patients with a high probability of false-negative results require confirmatory surgical staging. Talebian Yazdi et al. [34] evaluated 775 NSCLC patients with negative results by EBUS, EUS, or combined EBUS/EUS. Central location of the lung tumor, enlarged node on CT, and fluorodeoxyglucose avidity on PET were identified as predictors of false-negative results. In a retrospective study at a UK EBUS-TBNA center, lymph node standardized uptake value (SUV), SUV ratio between the primary tumor and lymph node, and heterogeneous echogenicity during sonographic assessment were independent predictors of false-negative results [35].
EBUS-TBNA FOR RESTAGING AFTER INDUCTION TREATMENT IN IIIA-N2 NSCLC
Although surgical resection after induction chemoradiotherapy in stage IIIA-N2 NSCLC showed no overall survival benefit in a phase III randomized trial [36], the role of surgery is still controversial because several reports have indicated a survival advantage in selected cases after surgical resection [37]. In a previous study of stage IIIA-N2 NSCLC, clearance of metastatic mediastinal lymph nodes (down-staging to N0/N1) after induction chemoradiotherapy was an independent prognostic factor in predicting the survival advantage of surgery [38]. Although restaging after induction treatment was conventionally performed using re-mediastinoscopy, it was not easy to detect residual metastatic lymph nodes because of mediastinal adhesion and fibrosis, with a disappointingly low sensitivity of 29% [39]. Instead of re-mediastinoscopy, endosonography was attempted to confirm the efficacy of restaging in IIIA-N2 NSCLC after induction therapy (Table 2). Herth et al. [40] reported that the diagnostic sensitivity of EBUS-TBNA for restaging was 76% after induction chemotherapy. In a retrospective study of patients undergoing induction chemotherapy with or without radiotherapy, the sensitivity and negative predictive value of EBUS-TBNA for restaging were 50% and 88%, respectively [41]. Combined EBUS-TBNA and EUS-B-FNA using a single ultrasound bronchoscope was attempted to restage patients with NSCLC after induction therapy and showed diagnostic sensitivity, negative predictive value, and accuracy of 67%, 73%, and 81%, respectively [42]. All patients in this study were confirmed by mediastinoscopy in cases of negative or uncertain results and metastatic lymph nodes were found in 18 of 69 patients with negative results by combined endosonography.

Outcomes of EBUS-TBNA for mediastinal restaging in patients with non-small cell lung cancer IIIA who underwent induction treatment
The diagnostic sensitivities of endosonography and re-mediastinoscopy for restaging were lower than those of initial staging in previous studies. Considering the better performance of mediastinoscopy when performed first, it appears reasonable to perform endosonography first for initial mediastinal staging and reserve mediastinoscopy for restaging. However, if endosonography is performed for restaging after induction therapy, negative results should be confirmed by mediastinoscopy considering the low negative predictive value of endosonography.
CONCLUSION
EBUS-TBNA is an essential modality for assessing the mediastinum of primary lung cancer with reduced invasiveness and improved safety. It should be performed with full understanding of technical aspects, knowledge of indications, and interpretation of results. Combined EBUS-TBNA and EUSFNA or EUS-B-FNA could increase the diagnostic yield of EBUS-TBNA and replace the previous gold standard, mediastinoscopy, in the initial mediastinal nodal staging of NSCLC. However, patients with a high probability of false-negative results on EBUS-TBNA, such as those with a centrally located lung tumor, enlarged lymph node on chest CT, or fluorodeoxyglucose avidity on PET/CT, require confirmatory mediastinoscopy. The diagnostic sensitivity of EBUS-TBNA for restaging after induction therapy in patients with stage IIIA-N2 NSCLC was lower than that of initial staging. It appears reasonable to perform EBUS-TBNA first for initial mediastinal staging and reserve mediastinoscopy for restaging after induction therapy.
Notes
No potential conflict of interest relevant to this article was reported.
