FGFR3-TACC3: A novel gene fusion in malignant melanoma
Article information
Abstract
Oncogenic gene fusions have been identified in many cancers, and many serve as druggable targets for therapy. Fibroblast growth factor receptor (FGFR) gene aberration is known to be associated with tumor progression and resistance to anticancer therapy. Here we report the first case of malignant melanoma harboring a FGFR3-TACC3 (transforming acidic coiled-coil containing protein 3) fusion, which appears to be a promising potential therapeutic target.
INTRODUCTION
Over the past decade, palliative treatment for melanoma has changed dramatically. The discovery of the oncogenic point mutation BRAF V600 and subsequent development of BRAF inhibitors and mitogen-activated protein kinase kinase (MEK) inhibitors have prolonged patient survival [1,2]. Imatinib or nilotinib for KIT mutated melanoma has shown promising response rates [3,4]. However, there are still many melanoma patients that do not have the option of targeted therapy, and the need for identification of a new therapeutic target is ever growing.
The fibroblast growth factor receptor (FGFR) family consists of four subtypes of transmembrane tyrosine kinase receptors (FGFR1-4) that play important roles in cell growth, differentiation, and angiogenesis [5]. Aberrant signaling through FGFR can occur through overexpression of receptors, ligands, and activating mutations of FGFR-containing translocations. FGFR gene fusions with multiple partners in a wide spectrum of tumors have been described. FGFR3-transforming acidic coiled-coil containing protein 3 (TACC3) fusions were first reported in glioblastoma and urothelial tumors [6,7]. This fusion proved to be an important therapeutic target in solid tumors and has supported the clinical development of several FGFR inhibitors and anti-FGFR drug conjugate antibodies [8]. Herein, we describe the first case of melanoma harboring a FGFR3-TACC3 fusion, revealed by a next-generation sequencing (NGS) assay.
CASE REPORT
A 66-year-old male presented to a clinic with blurred vision and right ocular swelling. An orbital computed tomography (CT) scan showed a mass with heterogeneous enhancement, occupying the right ethmoid sinus and nasal cavity, extending to the right medial orbital wall and skull base, causing bone destruction (Fig. 1).

Orbit computed tomography showing (A) a mass occupying the right ethmoid sinus and nasal cavity, (B) causing bony destruction at the skull base.
Endoscopic examination revealed a protruding mass with mucosal color change between the right middle turbinate and nasal septum. Serous discharge was drained upon biopsy via endoscopic sinus surgery (Fig. 2). The histopathologic finding showed diffuse large cell nest with prominent nucleoli and frequent plasmacytoid feature (Fig. 3) and positive immunoreactivity for S100 protein and HMB-45, which were consistent with malignant melanoma. The patient was referred to our clinic for systemic treatment in November 2017. Routine hematological and biochemical investigations were all within normal limits. Abdominal CT scan showed multiple metastatic masses in the perihepatic area and pelvic cavity (Fig. 4). Brain CT showed no definite evidence of brain metastasis. The patient started on pembrolizumab as a first-line chemotherapy, but experienced rapidly progressive disease and death due to cancer progression. A FGFR3-TACC3 fusion was identified in the tumor using a next-generation sequencing (NGS) platform (Oncomine Comprehensive Assay v3, ThermoFisher Scientific, Waltham, MA, USA; www.thermofisher.com).

Endoscopic examination revealed (A) a mass (arrows) between the right middle turbinate and nasal septum. (B) Serous discharge was drained upon biopsy.
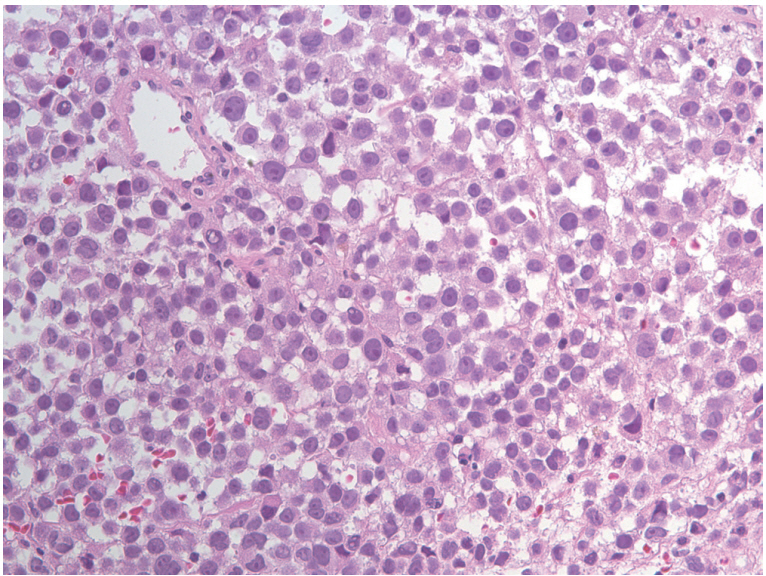
High magnification of resected specimen disclosed diffuse large cell nest with prominent nucleoli and frequent plasmacytoid feature (H&E stain, ×400).
DISCUSSION
The genetic aberrations most commonly found in malignant melanoma are BRAF, NRAS, and KIT mutations. BRAF mutations have been reported in 41% to 55% of metastatic melanoma. Most BRAF-activating mutations occurring in melanomas are at residue V600, usually V600E but occasionally V600K or other substitutions [9]. Inhibitors of MEK, a signaling molecule downstream of BRAF, potentiate the clinical activity of BRAF inhibitors in melanomas with BRAF V600 mutations. Multiple studies have demonstrated superior efficacy of BRAF/MEK inhibitors (dabrafenib/trametinib or vemurafenib/cobimetinib) combination therapy compared to BRAF monotherapy, with improved progression-free survival and response rate [1,2,10-14]. NRAS mutations, which have been reported in 15% to 20% of melanoma, are not yet druggable. KIT mutations, although rarer than BRAF mutations, are detected in 15% of mucosal and acral melanomas [15]. Studies have shown promising efficacy of imatinib and nilotinib, which are selective KIT inhibitors, for metastatic melanoma patients with KIT mutations [3,16,17]. However, a subset of patients has no targetable genetic aberrations and no chance to receive targeted therapies. Therefore, it is necessary to identify new targetable genetic aberrations in melanoma patients.
FGFRs consist of an extracellular domain encompassing three immunoglobulin-like domains (IgI, IgII, IgIII), followed by a transmembrane domain and two tyrosine kinase sub-domains, TKI and TKII. Fibroblast growth factors (FGFs) and the FGFR signaling pathway are vital in the management of tissue repair, wound healing, and tumor angiogenesis. It is crucial that FGF activity is tightly regulated to maintain homeostatic cellular proliferation [18].
After the first report of FGFR3-TACC3 gene fusion in glioblastoma in 2012, an FGFR3-TACC3 fusion was reported in numerous solid tumors including urothelial carcinoma, nonsmall cell lung cancer, thyroid, and uterine cervical carcinoma [8]. In the FGFR3-TACC3 fusion protein, the final exon at the C terminus of FGFR3 is replaced by TACC3, which mediates localization to the mitotic spindle. The mitotic localization of FGFR3-TACC3 fusion protein causes subsequent chromosomal segregation defects, resulting in aneuploidy [6,19]. Further, a cell line study showed a 2-fold increase in tyrosine kinase phosphorylation on the FGFR3-TACC3 fusion protein compared to that of FGFR3 wild type [20]. Deregulation of the FGF-FGFR signaling axis has been implicated in oncogenesis, tumor progression, and resistance to anticancer therapy across many tumor types [19]. Along with preclinical results, FGFR3-TACC3 fusion appears to be a promising target of inhibition. In a phase I trial of JNJ42756493, an oral pan-FGFR inhibitor, there were three out of four confirmed partial responders (two urothelial cancer, one glioblastoma) who harbored the FGFR3-TACC3 fusion. Tumor shrinkage was also seen in a patient with adrenal carcinoma with predominant FGFR3-TACC3 translocation [21]. More early-phase studies are needed. A phase I/II trial of AZD4547, an oral selective inhibitor of FGFR 1, 2, and 3 receptor tyrosine kinase, is currently being investigated in patients with recurrent glioma expressing FGFR3-TACC3 or FGFR1-TACC1 fusion (NCT02824133). A phase II trial of BGJ398, a selective pan-FGFR kinase inhibitor, has been conducted in recurrent glioblastoma patients with FGFR3-TACC3, FGFR1-TACC1 fusion, or activating mutation in FGFR1, 2 or 3 (NCT01975107).
To the best of our knowledge, this is the first report to identify an FGFR3-TACC3 fusion in malignant melanoma. This rare gene fusion event was identified in metastatic mucosal melanoma arising from nasal cavity at diagnosis and the clinical course was rapidly deteriorating despite of pembrolizumab. Given the aggressive and poor survival of mucosal melanoma, a better understanding of genetic changes is needed to lay the foundation for development of more effective therapeutic agents. This report provides proof of concept that treatment with an FGFR inhibitor can provide clinical benefit in malignant melanoma cases carrying FGFR3-TACC3 translocation, in agreement with results observed in other malignancies. Further, our finding highlights the importance of a comprehensive genomic profiling approach able to detect all classes of genomic alterations, including uncommon gene fusions, to reveal potentially targetable somatic alterations to inform new strategies for patients with limited therapeutic options.
Notes
No potential conflict of interest relevant to this article was reported.
