 |
 |
- Search
| Precis Future Med > Volume 1(1); 2017 > Article |
|
Abstract
Recently, monoclonal antibodies targeting the programmed death 1 (PD-1)/PD ligand 1 (PD-L1) pathway have dramatically changed the treatment paradigm for non-small cell lung cancer (NSCLC). Several PD-1/PD-L1 inhibitors have been validated as second-line therapies for NSCLC, and are now being actively investigated as first-line therapies. In this paper, current data and future strategies for their use in first-line therapy are reviewed. There are five PD-1/PD-L1 inhibitors in development for NSCLC. Among them, three drugs (nivolumab, pembrolizumab, and atezolizumab) have been approved as second-line therapies for NSCLC in the United States. As a first-line therapy, pembrolizumab successfully showed superiority over platinum-based doublet chemotherapy in a strongly PD-L1-positive NSCLC group, while nivolumab failed to show efficacy as a first-line therapy. Currently, five PD-1/PD-L1 inhibitors are being tested as a first-line therapy for NSCLC in more than 10 phase III studies, with various study designs. Although it is not yet clear whether PD-1/PD-L1 inhibitors should be used as a monotherapy or in combination according to PD-L1 expression in first-line therapies, these promising drugs may play an important role in clinical practice as a first-line therapy for NSCLC in the near future.
Immune checkpoint inhibitors have been evaluated for advanced non-small cell lung cancer (NSCLC) in many trials. In particular, programmed death 1 (PD-1)/PD ligand 1 (PD-L1) inhibitors have been researched extensively in patients with advanced NSCLC. PD-1 is a 50- to 55-kDa type I transmembrane glycoprotein composed of an immunoglobulin variable (IgV)-type extracelluar domain, and it is expressed on T cells in thymus and induced on peripheral T and B cells upon activation. PD-L1 is type I transmembrane glycoproteins composed of IgC- and IgV-type extracellular domains, and it is expressed on various normal tissues such as placenta, heart, lung, and liver as well as lymphoid and tumor tissues. The interaction between PD-1 and PD-L1 prevents autoimmunity and antitumor effects by inhibiting the activation of autoreactive lymphocytes. There are five PD-1/PD-L1 inhibitors in development for NSCLC that are actively competing with regard to efficacy for NSCLC: PD-1 inhibitors (nivolumab and pembrolizumab) and PD-L1 inhibitors (atezolizumab, durvalumab, and avelumab).
The promising data from several pivotal trials have made three drugs (nivolumab, pembrolizumab, and atezolizumab) be approved as second-line therapies for NSCLC in the United States. However, study data and approval indications vary somewhat, especially in the predictive role of PD-L1 protein expression among these compounds.
There is growing interest in the potential of PD-1/PD-L1 inhibitors for use as a first-line therapy, as their efficacy and tolerability has been proven in second (or beyond)-line therapy. Currently, for advanced NSCLC not harboring oncogenic epidermal growth factor receptor (EGFR) or anaplastic lymphoma kinase (ALK) mutations, platinum-based doublet chemotherapy is the standard therapy with a median progression-free survival (PFS) of 4 to 6 months and median overall survival of 8 to 10 months [1]. As of January 2017, the efficacy of PD-1/PD-L1 inhibitors as a first-line therapy has already been presented in two phase III trials, though they had contradictory clinical results [2,3]. In addition, there are more than 10 ongoing phase III trials, with most data expected to be presented within a couple of years. Therefore, a review of clinical data that have been presented will help us predict upcoming changes in the treatment paradigm for NSCLC.
Several pivotal trials evaluated the efficacy of PD-1/PD-L1 inhibitors for NSCLC as a second-line therapy (Table 1). Based on these data, nivolumab, pembrolizumab, and atezolizumab were approved for second-line use in NSCLC in the United States. According to the clinical trials described below, all NSCLC patients are allowed nivolumab and atezolizumab regardless of PD-L1 expression, and patents with PD-L1-positive (≥1% PD-L1 expression) NSCLC are allowed pembrolizumab. In South Korea, nivolumab and pembrolizumab are approved for patients with NSCLC, though they have not yet been reimbursed by the government-operated reimbursement system.
In the CheckMate 017 and 057 trials, second-line nivolumab was compared with docetaxel in patients with squamous and non-squamous NSCLC, respectively [4,5]. For squamous NSCLC (CheckMate 017), nivolumab was superior to docetaxel in terms of both PFS (hazard ratio [HR], 0.62; 95% confidence interval [CI], 0.47 to 0.81; P<0.001) and overall survival (HR, 0.59; 95% CI, 0.44 to 0.79; P<0.001), irrespective of PD-L1 expression assessed using the Dako platform (28-8 PD-L1 primary Ab) [4]. For non-squamous NSCLC (CheckMate 057), however, nivolumab was superior to docetaxel in terms of overall survival (HR, 0.73; 95% CI, 0.59 to 0.89; P=0.002), while PFS were not different between the two arms (HR, 0.92; 95% CI, 0.77 to 1.11; P=0.39) [4]. In addition, on the contrary data of CheckMate 017, PD-L1 expression affected the efficacy of nivolumab: in subgroup analysis according to PD-L1 expression, nivolumab led to longer PFS than docetaxel in PD-L1-positive subgroups defined by various PD-L1 cutoff values (1%, 5%, and 10%).
The efficacy of pembrolizumab was evaluated in relation to PD-L1 expression in a phase I study, KEYNOTE-001 [6]. The study recommended that tumor tissues for immunohistochemistry tests of PD-L1 expression be biopsied within 6 months before enrollment, and a PD-L1 clinical trial assay (22C3 Ab, Dako, Carpinteria, CA, USA) was also validated with comparison with another prototype assay (22C3 Ab, Qualteck, Goleta, CA, USA). With strict controls for PD-L1 testing, the objective response rate (ORR) for pembrolizumab increased proportionally with PD-L1 expression in tumor cells (TCs): ORRs were 8%, 13%, 19%, 30%, and 45% in subgroups with PD-L1 expression <1%, 1% to 24%, 25% to 49%, 50% to 74%, and 75% to 100%, respectively. In a phase II/III trial, KEYNOTE-010, two doses of pembrolizumab (10 or 3 mg/kg every 3 weeks) were compared with docetaxel as a second-line therapy in patients with PD-L1-positive NSCLC [7]. This study required new biopsies for PD-L1 testing, but allowed archival tissue if repeated biopsy was risky. As a result, 44% of enrolled subjects were tested for PD-L1 expression using archival tissue. Among 2,222 screened patients for PD-L1 expression, 842 (66.4%) had PD-L1 expression ≥1% and 633 (28.5%), PD-L1 ≥50%. The co-primary endpoints were PFS and overall survival in both the PD-L1 ≥1% and ≥50% groups, respectively. In the PD-L1 ≥1% group (total study population), high-dose pembrolizumab (10 mg/kg) was significantly superior to docetaxel in terms of both PFS (HR, 0.79; 95% CI, 0.66 to 0.94; P=0.004) and overall survival (HR, 0.61; 95% CI, 0.49 to 0.75; P<0.0001), while low-dose pembrolizumab (2 mg/kg) was significantly superior to docetaxel only in terms of overall survival (HR, 0.71; 95% CI, 0.58 to 0.88; P=0.0008), and not in PFS (HR, 0.88; 95% CI, 0.74 to 1.05; P=0.07). In the PD-L1 ≥50% group, the median PFS of high- and low-dose pembrolizumab was 5.2 months (HR, 0.59; 95% CI, 0.45 to 0.78; P<0.0001) and 5.0 months (HR, 0.59; 95% CI, 0.44 to 0.78; P=0.0001), respectively, and the median overall survival of high- and low-dose pembrolizumab was 17.3 months (HR, 0.50; 95% CI, 0.36 to 0.70; P<0.0001) and 14.9 months (HR, 0.54; 95% CI, 0.38 to 0.77; P=0.0002), which was significantly superior to docetaxel.
Atezolizumab was compared with docetaxel as a second- or third-line therapy in phase II (POPLAR) and phase III (OAK) trials, respectively [8,9]. Unlike studies of nivolumab and pembrolizumab, PD-L1 expression (SP263 Ab, Ventana) was evaluated in tumor-infiltrating immune cells (ICs) as well as in TCs. PD-L1 expression in TCs was categorized as PD-L1 expression ≥50% (TC3), ≥5% but <50% (TC2), ≥1% but <5% (TC1), and <1% (TC0). In ICs it was categorized as PD-L1 expression ≥10% (IC3), ≥5% but <10% (IC2), ≥1% but <5% (IC1), and <1% (IC0). As a result of the combination of TC and IC scores, patients were divided into four groups: (1) TC3 or IC3; (2) TC2/3 or IC2/3; (3) TC1/2/3 or IC1/2/3; and (4) TC0 and IC0. Among the screened POPLAR population [8], the proportions of (TC3 or IC3), (TC2/3 or IC2/3), (TC1/2/3 or IC1/2/3), or (TC0 or IC0) were 16%, 37%, 68%, and 32%, respectively. In the POPLAR trial, the overall survival of the atezolizumab arm was superior to that of docetaxel arm (HR, 0.73; 95% CI, 0.53 to 0.99; P=0.040), showing a close relationship between the efficacy of atezolizumab with PD-L1 expression and no difference in survival between two arms in a subgroup of TC0 and IC0 [8]. In the OAK trial, the superiority of atezolizumab over docetaxel was confirmed again in terms of overall survival in the total population (HR, 0.73; 95% CI, 0.62 to 0.87; P=0.0003) [9]. Unlike the POPLAR trial, however, its superior efficacy was consistently significant around all PD-L1 subgroups including a subgroup of TC0 and IC0, though the favorable survival outcome of atezolizumab was more obvious in strong PD-L1 expression groups compared to weak or no PD-L1 expression groups. Interestingly, as in the CheckMate 057 study (nivolumab vs. docetaxel for non-squamous NSCLC), the PFS curves of the two study arms crossed in the middle of the follow-up period and there was no significant difference in PFS between the two arms. However, in a similar pattern with the CheckMate 057 (nivolumab vs. doxetaxel for squamous NSCLC), the PFS of nivolumab was superior to that of docetaxel in strong PD-L1 expression groups (TC2/3 or IC2/3, TC3 or IC3).
In the single-arm phase II ATLANTIC trial, durvalumab was evaluated as third or beyond line therapy [10]. In total, 1,122 patients were screened for PD-L1 expression (SP263 Ab, Ventana), and approximately 33% and 16% of those patients exhibited ≥25% and ≥90% PD-L1 expression, respectively. The ORR increased according to PD-L1 expression: 7.5% in the PD-L1 <25% group, 16.4% in the PD-L1 ≥25% group, and 30.9% in the PD-L1 ≥90% group. In the phase III ARCTIC trial, previously-treated NSCLC patients will be categorized as PD-L1 (+) (expression ≥25%) and (–), and will be randomized into durvalumab or standard of chemotherapy in the PD-L1-positive group and into durvalumab plus tremelimumab, durvalumab monotherapy, tremelimumab monotherapy, or standard chemotherapy in the PD-L1-negative group, respectively (NCT02352948) [11]. This study completed enrollment and its data are expected to be released within a year.
Nivolumab was evaluated as a first-line therapy for NSCLC in the phase III CheckMate 026 trial [3]. In total, 541 patients with advanced NSCLC expressing PD-L1 in ≥1% of TCs were randomized into nivolumab 3 mg/kg every 2 weeks or platinum-based doublet chemotherapy, and crossover from chemotherapy to nivolumab was allowed. The PFS, the primary endpoint, in a group with PD-L1 ≥5% did not vary between the two groups: the median PFS for nivolumab and chemotherapy were 4.2 and 5.9 months, respectively (HR, 1.15; 95% CI, 0.91 to 1.45; P=0.2511). This result was unexpected and disappointing for nivolumab, because, in the CheckMate 057 trial, nivolumab was superior to docetaxel in PD-L1-positive subgroups defined by various PD-L1 cutoff levels (1%, 5%, and 10%) [5]. In the CheckMate 057 trial (nivolumab vs. docetaxel as a second-line therapy), the median PFS for nivolumab and docetaxel were, respectively, 5.0 and 3.8 months (HR, 0.54; 95% CI, 0.39 to 0.76) in the ≥5% PD-L1 expression group, and 4.2 and 4.5 months (HR, 0.70; 95% CI, 0.53 to 0.94), respectively, in the ≥10% PD-L1 expression group. Comparing the two trials, median PFS for first-line nivolumab (CheckMate 026) was not arithmetically superior to that of second-line nivolumab (CheckMate 057), while the median PFS for chemotherapy increased considerably. This comparison of PFS between two studies suggests that the failure of the CheckMate 026 trial lies in no increase in the efficacy of nivolumab according to the line of therapy, while the efficacy of the comparator (chemotherapy) improved significantly as the chemotherapy regimen was switched to platinum-doublet chemotherapy from docetaxel monotherapy.
Pembrolizumab was also evaluated as a first-line therapy for NSCLC in the KEYNOTE-024 trial. Untreated patients with advanced NSCLC harboring neither EGFR mutation nor ALK rearrangement were eligible for this phase III trial. Among 1,653 subjects screened for PD-L1, 500 (30.2%) had tumors expressing PD-L1 ≥50%, among which 305 were enrolled and randomized into pembrolizumab 200 mg every 3 weeks (n=154) and platinum-doublet chemotherapy (n=151). The ORRs for pembrolizumab and chemotherapy were 44.8% and 27.8%, respectively. The PFS, the primary endpoint, for pembrolizumab was significantly longer than chemotherapy (median PFS: 10.3 months vs. 6.0 months; HR, 0.50; 95% CI, 0.37 to 0.68). More interestingly, the overall survival of the pembrolizumab arm was also superior to the chemotherapy arm (HR, 0.60; 95% CI, 0.41 to 0.89), even when 43.7% of the chemotherapy arm were crossed over to pembrolizumab arm at disease progression. Based on this encouraging data, pembrolizumab was recently approved as a first-line therapy for patients with PD-L1-positive (≥50% expression) NSCLC harboring neither EGFR mutation nor ALK gene rearrangement.
Though the reason for the contradictory result of the two PD-1 inhibitor studies (CheckMate 026 and KEYNOTE-024) cannot be defined conclusively, it might be in most part attributed to the different method of patient selection between two studies. CheckMate 026 and KEYNOTE-024 used PD-L1 28-8 antibody (Dako, Carpinteria, CA, USA) and PD-L1 22C3 antibody (Dako), respectively, in the selection of PD-L1-positive patients. Compared with CheckMate 026, KEYNOTE-024 strongly recommended newly biopsied tissue for PD-L1 testing and required higher level of PD-L1 positivity (≥50% of TCs), leading to more strict enrichment for patients who possibly more likely benefit from pembrolizumab. In addition, the PD-L1 testing by KEYNOTE-024 had been validated as the predictive marker from the phase I study, KEYNOTE-001 [6].
In the recently released single-arm phase II BIRCH trial, first-line atezolizumab was evaluated [12]. Total 138 patients with PD-L1 expression (TC 2/3 or IC 2/3) were treated with atezolizumab 1,200 mg every 3 weeks. Like previously reported atazolizumab studies, the response rate was better in a subgroup with stronger PD-L1 expression (TC3 or IC3) than in a subgroup of PD-L1 expression with TC2 or IC2 (34% vs. 18%). The median PFS for this group (TC2/3 or IC2/3) was 7.3 months, which was longer than that (4.1 months) of the subgroup with the same PD-L1 expression (TC2/3 or IC2/3) in the OAK trial [9].
As shown in the CheckMate 026 trial, PD-1/PD-L1 inhibitor monotherapy appears unlikely to beat platinum-based doublet chemotherapy as a first-line therapy for NSCLC, especially in an unselected or poorly selected population. Therefore, a strategy to improve the efficacy of first-line PD-1/PD-L1 inhibitor therapy was invented. Combination therapy with cytotoxic chemotherapy or cytotoxic T-lymphocyte-associated protein 4 (CTLA4) inhibitors, another type of immune checkpoint inhibitor, such as ipilimumab or tremelimumab, has been investigated in many trials.
In the CheckMate 012 trial, nivolumab (3 mg/kg every 2 weeks) was combined with ipiliumab (1 mg/kg every 6 or 12 weeks) [13,14]. The ORR of the combination therapy was higher than that of nivolumab monotherapy (43% vs. 23%), and PFS increased compared with nivolumab monotherapy (10.4 to 13.2 months vs. 3.5 months). However, there are concerns regarding increased toxicity, especially immune-related toxicity, which is associated with dual immunotherapy. Grade 3/4 treatment-related toxicity was more frequent in the combined nivolumab/ipilimumab therapy compared with nivolumab monotherapy (31% to 42% vs. 19%). Dual immunotherapy is currently being investigated in a large phase III trial as a first-line therapy compared with platinum-based doublet chemotherapy (CheckMate 227, NCT02477826) (Fig. 1).
Another strategy for combined therapy is PD-1/PD-L1 inhibitor plus cytotoxic chemotherapy. In the KEYNOTE-021 trial, EGFR/ALK-negative, untreated NSCLC patients were randomized into pemetrexed/carboplatin plus pembrolizumab or pemetrexed/carboplatin only [15]. The response rates were 55% and 29%, respectively, for combination therapy and chemotherapy only (P=0.0016). Interestingly, in subgroup analysis according to PD-L1 expression, the response rates were 80% in the ≥50% PD-L1 expression subgroup and 57% in the <1% PD-L1 expression subgroup. The superior efficacy of combined therapy even in PD-L1-negative patients might have prevented early progression, which has happened frequently when PD-1/PD-L1 inhibitor is used as a monotherapy [4,5,7-9,16]. More interestingly, discontinuation rates due to adverse events were similar for the combination therapy arm and the chemotherapy only arm (10% vs. 13%). This promising data regarding the first-line combination regimen of pembolizumab plus pemetrexed/carboplatin are now being validated in a phase III trial (KEYNOTE-189, NCT02578680) (Fig. 1), and this study is also expected to be released within a year.
More than 10 ongoing phase III trials are evaluating the role of PD-1/PD-L1 inhibitors is being evaluated as a first-line therapy for NSCLC (Fig. 1). It would be useful to be able to predict the clinical outcomes of these studies. Some studies have PFS, overall survival or both PFS and overall survival as their primary endpoints. Because of the unique pattern of clinical activity of PD-1/PD-L1 inhibitors, the PFS of PD-1/PD-L1 inhibitors was not superior to that of chemotherapy, while PD-1/ PD-L1 inhibitors showed significant efficacy in terms of overall survival in previously treated NSCLC patients [5,8,9]. Among the studies shown in Fig. 1, seven trials had PFS as their only primary endpoint, of which three studies directly compared PD-1/PD-L1 inhibitors monotherapy with platinum-doublet chemotherapy. Although these trials included only PD-L1-positive patients, PD-1/PD-L1 inhibitor monotherapy is unlikely to beat platinum-based doublet chemotherapy in terms of PFS in a poorly or loosely enriched group, as shown in the CheckMate 026 trial. Furthermore, with the recent regulatory approval of some PD-1/PD-L1 inhibitors as second-line therapies in many countries, studies of first-line PD-1/PD-L1 inhibitors should additionally consider crossover as the compounding factor, which could make it more difficult to meet the primary endpoint, overall survival.
In current ongoing trials, PD-1/PD-L1 inhibitors are tested as a monotherapy or in combination therapy with dual immune checkpoint inhibitors (nivolumab/ipilimumab or durvalumab/tremelimumab) or chemotherapy. It is difficult to determine which strategy is the most promising strategy as a first-line therapy for NSCLC, because data thus far are preliminary. However, it is possible these three strategies may be used for different subsets of populations in the future. Based on the KEYNOTE-024 trial, a biomarker-defined subset of EGFR/ALK-negative and PD-L1-strong positive (e.g., PD-L1 ≥50% expression on 22C3 Ab) indicates a good candidate for first-line PD-1/PD-L1 inhibitor treatment as a monotherapy. For patients with PD-L1-weak positive or -negative tumors, a first-line combination therapy should be considered based on the CheckMate 227 and KEYNOTE-021 trial [14,15], although data from large randomized trials are needed to confirm this. In addition, when considering the combination therapy, the physicians should keep in mind about additional toxicities and should be more alert for these events. In addition, for those with low PD-L1 expression, PD-1/PD-L1 inhibitors can be reserved for second (or beyond)-line therapy following platinum-based chemotherapy, based on data drawn from many second-line therapy trials [4,5,7-9].
Currently, many studies on PD-1/PD-L1 inhibitors as a first-line therapy for NSCLC are being conducted, and their data will be published within the next few years. PD-1/PD-L1 inhibitors are gaining ground as a first-line therapy in the form of a monotherapy or in combination with other agents. Accordingly, immunohistochemistry testing to evaluate PD-L1 expression will become an important biomarker test for patients with NSCLC at the time of diagnosis. While PD-L1 expression is thus far the most reliable and readily available predictive marker, it is not a perfect biomarker due to a number of shortcomings such as the various PD-L1 assays and different PD-L1 cutoff values used, and the substantial clinical benefit from PD-1/PD-L1 inhibitors seen even in patients with PD-L1-negative tumors. The relatively unreliable predictive marker is the most challenging or weak point in the application of PD-1/PD-L1 inhibitors into clinical practice, compared with small molecule inhibitors such as EGFR or ALK inhibitors. As a result, more efforts to investigate useful biomarkers, such as mutation burden, neo-antigens, and tumor infiltration lymphocytes, are needed. Ultimately, more accurate predictive biomarkers will allow clinicians to identify a subset of patients who are most likely to benefit from PD-1/PD-L1 inhibitors alone or in combination with chemotherapy or CTLA-4 inhibitors.
Fig. 1.
Ongoing studies of first-line programmed death 1 (PD-1)/PD ligand 1 (PD-L1) inhibitors: more than 10 phase III studies comparing PD-1/PD-L1 inibitors with chemotherapy are currently ongoing, and the results are expected to be released within a few years. NSCLC, non-small cell lung cancer; CTx, chemotherapy; SOC, standard of chemotherapy; OS, overall survival; PFS, progression-free survival; SqCC, squamous cell carcinoma.
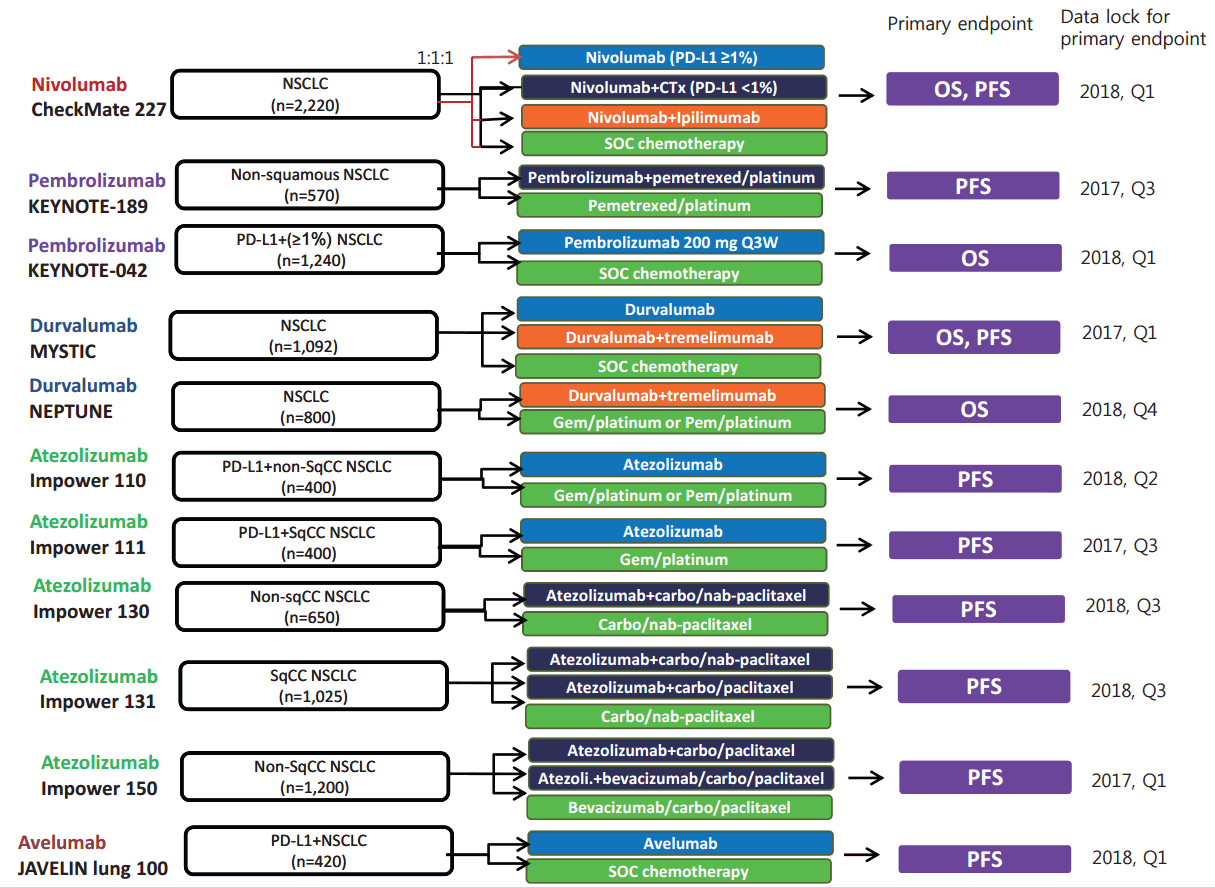
Table 1.
PD-1/PD-L1 inhibitor trials in the treatment of NSCLC as a second (or beyond)-line therapy
| Drug | Study | Study phase | Population | Comparator | Response rate | Median PFS (HR, 95% CI) | Median OS (HR, 95% CI) |
|---|---|---|---|---|---|---|---|
| Nivolumab | CheckMate 017 [4] | III | Squamous NSCLC | Docetaxel | 20% vs. 9% (P = 0.008) | 3.5 mo (0.62, 0.47–0.81) | 9.2 mo (0.59, 0.44–0.79) |
| CheckMate 057 [5] | III | Non-squamous NSCLC | Docetaxel | 19% vs. 12% (P = 0.02) | 2.3 mo (0.92, 0.77–1.11) | 12.2 mo (0.73, 0.59–0.89) | |
| Pembrolizumab | KEYNOTE-001 [6] | I | NSCLC | Single arm | 19.4% (overall) | 3.7 mo (NA) | 12.0 mo (NA) |
| 8.1% (PS < 1%) | |||||||
| 12.9% (PS 1%–24%) | |||||||
| 19.4% (PS 25%–49%) | |||||||
| 29.6% (PS 50%–74%) | |||||||
| 45.4% (PS 75%–100%) | |||||||
| KEYNOTE-010 [7] | II/III | PD-L1 (PS ≥1%) NSCLC | Docetaxel | 18% vs. 9% (P = 0.0005)a) | 4.0 mo (0.79, 0.66–0.94)a) | 12.7 mo (0.61, 0.49–0.75)a) | |
| 18% vs. 9% (P = 0.002)b) | 4.0 mo (0.88, 0.74–1.05)b) | 10.4 mo (0.71, 0.58–0.88)b) | |||||
| PD-L1 (PS ≥50%) NSCLC | Docetaxel | 29% vs. 8% (P<0.0001)a) | 5.2 mo (0.59, 0.45–0.78)a) | 17.3 mo (0.50, 0.36–0.70)a) | |||
| 30% vs. 8% (P < 0.0001)b) | 5.0 mo (0.59, 0.44–0.78)b) | 14.9 mo (0.54, 0.38–0.77)b) | |||||
| Atezolizumab | POPLAR [8] | II | NSCLC | Docetaxel | 14.6% vs. 14.7% (NS) | 2.7 mo (0.94, 0.72–1.23) | 12.6 mo (0.73, 0.53–0.99) |
| OAK [9] | III | NSCLC | Docetaxel | 14% vs. 13% (NS) | 2.8 mo (0.95, 0.82–1.10) | 13.8 mo (0.73, 0.62–0.87) | |
| Durvalumab | ATLANTIC [10] | II | NSCLC | Single arm | 16.4% (PD-L1 ≥ 25%) | 3.3 mo (PD-L1 ≥ 25%) | 10.9 mo (PD-L1 ≥ 25%) |
REFERENCES
1. Scagliotti GV, Parikh P, von Pawel J, Biesma B, Vansteenkiste J, Manegold C, et al. Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naive patients with advanced-stage non-small-cell lung cancer. J Clin Oncol 2008;26:3543–51.


2. Reck M, Rodriguez-Abreu D, Robinson AG, Hui R, Csoszi T, Fulop A, et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med 2016;375:1823–33.


3. Socinski M, Creelan B, Horn L, Reck M, Paz-Ares L, Steins M, et al. NSCLC, metastaticCheckMate 026. A phase 3 trial of nivolumab vs investigator’s choice (IC) of platinum-based doublet chemotherapy (PT-DC) as first-line therapy for stage iv/recurrent programmed death ligand 1 (PD-L1): positive NSCLC. Ann Oncol 2016;27(Suppl 6):LBA7-PR.

4. Brahmer J, Reckamp KL, Baas P, Crino L, Eberhardt WE, Poddubskaya E, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med 2015;373:123–35.



5. Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med 2015;373:1627–39.



6. Garon EB, Rizvi NA, Hui R, Leighl N, Balmanoukian AS, Eder JP, et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med 2015;372:2018–28.


7. Herbst RS, Baas P, Kim DW, Felip E, Perez-Gracia JL, Han JY, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet 2016;387:1540–50.


8. Fehrenbacher L, Spira A, Ballinger M, Kowanetz M, Vansteenkiste J, Mazieres J, et al. Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): a multicentre, open-label, phase 2 randomised controlled trial. Lancet 2016;387:1837–46.


9. Rittmeyer A, Barlesi F, Waterkamp D, Park K, Ciardiello F, von Pawel J, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet 2017;389:255–65.


10. Garassino M, Vansteenkiste J, Kim JH, Lena H, Mazieres J, Powderly J, et al. PL04a.03: Durvalumab in ≥3rd-line locally advanced or metastatic, EGFR/ALK wild-type NSCLC: results from the phase 2 ATLANTIC study. J Thorac Oncol 2017;12(1 Suppl):S10–1.

11. Planchard D, Yokoi T, McCleod MJ, Fischer JR, Kim YC, Ballas M, et al. A phase III study of durvalumab (MEDI4736) with or without tremelimumab for previously treated patients with advanced NSCLC: rationale and protocol design of the ARCTIC study. Clin Lung Cancer 2016;17:232–6. e1.


12. Garassino M, Rizvi N, Besse B, Janne P, Christoph D, Peters S, et al. OA03.02 Atezolizumab as 1L therapy for advanced NSCLC in PD-L1-selected patients: updated ORR, PFS and OS data from the BIRCH study. J Thorac Oncol 2017;12(1 Suppl):S251–2.

13. Gettinger S, Rizvi NA, Chow LQ, Borghaei H, Brahmer J, Ready N, et al. Nivolumab monotherapy for first-line treatment of advanced non-small-cell lung cancer. J Clin Oncol 2016;34:2980–7.



14. Hellmann MD, Rizvi NA, Goldman JW, Gettinger SN, Borghaei H, Brahmer JR, et al. Nivolumab plus ipilimumab as first-line treatment for advanced non-small-cell lung cancer (CheckMate 012): results of an open-label, phase 1, multicohort study. Lancet Oncol 2017;18:31–41.



15. Langer CJ, Gadgeel SM, Borghaei H, Papadimitrakopoulou VA, Patnaik A, Powell SF, et al. Carboplatin and pemetrexed with or without pembrolizumab for advanced, non-squamous non-small-cell lung cancer: a randomised, phase 2 cohort of the open-label KEYNOTE-021 study. Lancet Oncol 2016;17:1497–508.










